The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma - PubMed (original) (raw)
The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma
Przemyslaw Juszczynski et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Classical Hodgkin lymphomas (cHLs) contain small numbers of neoplastic Reed-Sternberg (RS) cells within an extensive inflammatory infiltrate that includes abundant T helper (Th)-2 and T regulatory (Treg) cells. The skewed nature of the T cell infiltrate and the lack of an effective host antitumor immune response suggest that RS cells use potent mechanisms to evade immune attack. In a screen for T cell-inhibitory molecules in cHL, we found that RS cells selectively overexpressed the immunoregulatory glycan-binding protein, galectin-1 (Gal1), through an AP1-dependent enhancer. In cocultures of activated T cells and Hodgkin cell lines, RNAi-mediated blockade of RS cell Gal1 increased T cell viability and restored the Th1/Th2 balance. In contrast, Gal1 treatment of activated T cells favored the secretion of Th2 cytokines and the expansion of CD4+CD25high FOXP3+ Treg cells. These data directly implicate RS cell Gal1 in the development and maintenance of an immunosuppressive Th2/Treg-skewed microenvironment in cHL and provide the molecular basis for selective Gal1 expression in RS cells. Thus, Gal1 represents a potential therapeutic target for restoring immune surveillance in cHL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
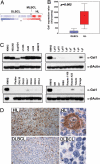
Gal1 is overexpressed in cHL cell lines and primary tumors. Relative Gal1 mRNA abundance (A and B) and protein expression (C) in a panel of LBCL and cHL cell lines are shown. (A) Gal1 expression profiles of DLBCL, MLBCL, and cHL cell lines. Color scale at the bottom indicates relative expression ± SEM. Red connotes high-level expression; blue indicates low-level expression. (B) Median expression of Gal1 (boxes) in LBCL vs. cHL cell lines ± 25–75 percentile (bars) and ± range (whiskers). (C) Respective cHL cell lines (KMHZ, HDLM2, SupHD1, L1236, L540, L428, and HD-MY-Z), the MLBCL cell line (Karpas 1106), and DLBCL cell lines (all others). (D) Gal1 immunohistochemistry (IHC). Gal1 IHC of a representative primary cHL (Upper) and DLBCL (Lower) are shown. [Original magnifications: ×40 (Upper) and ×400 (Lower).]
Fig. 2.
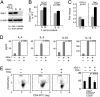
Gal1 transcription is regulated by an AP1-dependent enhancer. (A) Analysis of the AP1-dependent Gal1 enhancer. The previously described Gal1 promoter (21) and putative enhancer element including or lacking the predicted AP1-binding site (represented by a black bar) were cloned into a luciferase reporter vector, transiently transfected into cHL HD-MY-Z cells, and assayed for luciferase activities. Representative luciferase activities from three independent experiments are normalized to Renilla luciferase activity and are presented as bars ± SD. (B) Selective activity of the Gal1 enhancer. Classical HL, DLBCL, and fibroblast lines were transfected with either the Gal1 promoter-only vector (pGL3-Gal1−403 +67-Luc) or the promoter–enhancer construct (pGL3-Gal1403 +67-Luc-e1346–1746) and assessed as in A for their respective luciferase activities. (C) AP-1 dependence of the Gal1 enhancer in electrophoretic mobility shift assays. Nuclear extracts from DLBCL cell lines (DHL4, DHL7, and Toledo) or cHL cell lines (HD-MY-Z, L428, and SupHD1) were incubated with WT or mutant 32P-labeled, double-stranded DNA probe corresponding to AP1-binding site in Gal1 enhancer. Specific, unlabeled competitor and antibodies against c-Jun or β-actin (control) were included in certain assays as indicated. The gel-shift band corresponding to the probe–protein complex is indicated with an arrow, and supershift bands corresponding to the probe–protein–antibody complex are noted with asterisks. (D) c-Jun dependence of the Gal1 enhancer. HD-MY-Z cells were cotransfected with the Gal1 promotor-only vector or the Gal1 promotor–enhancer construct with either the dominant-negative c-Jun (c-Jun-DN) construct (c-Jun-DN) or empty vector. Luciferease activities were determined as in A. (E) AP1 inhibition decreases Gal1 transcript abundance. HD-MY-Z cells were transfected with either c-Jun-DN or empty vector. Thereafter, relative Gal1 mRNA abundance was assessed by real-time QPCR.
Fig. 3.
Gal1 confers immune privilege to cHL RS cells by favoring the expansion of Th2 cells and Treg cells. (A) RNAi-mediated blockade of Gal1 expression in the cHL HD-MY-Z cell line. HD-MY-Z cells were transduced with pSIREN-RetroQ vector encoding Gal1-specific shRNA (Gal1 shRNA, G) or scrambled control shRNA (SCR shRNA, S) and analyzed thereafter for Gal1 protein expression. (B) Viability of total (CD3+) and CD4+ T cells cocultured with Gal1 shRNA cHL or control SCR shRNA cHL cells. After coculture, T cell viability was assessed by using triple-color annexin-V, CD3, and CD4 flow cytometry. (C) Relative abundance of the Th1- and Th2-specific transcription factors, T-bet and GATA-3, in CD4+ cells from the Gal1 shRNA and SCR shRNA (control) cHL/T cell cocultures. (D) Th2 cytokine production by Gal1-treated T cells. Activated T cells were either untreated or treated with rGal1 in the presence or absence of TDG. Th2 cytokine (IL-4, IL-5, IL-10, and IL-13) production was then assessed by cytometric bead arrays. (E) Treg cell abundance in Gal1-treated T cells. Activated T cells were cultured in the presence of rGal1 or rGal1+TDG or left untreated. The percentage of CD4+CD25+FOXP3+ T cells was then assessed by triple-color flow cytometry. The histograms (Left) are representative of three separate experiments averaged to obtain the percent of CD4+CD25highFOXP3+ cells in the bar graph (Right).
Similar articles
- AP1-dependent galectin-1 expression delineates classical hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features.
Rodig SJ, Ouyang J, Juszczynski P, Currie T, Law K, Neuberg DS, Rabinovich GA, Shipp MA, Kutok JL. Rodig SJ, et al. Clin Cancer Res. 2008 Jun 1;14(11):3338-44. doi: 10.1158/1078-0432.CCR-07-4709. Clin Cancer Res. 2008. PMID: 18519761 - Expression of PIM kinases in Reed-Sternberg cells fosters immune privilege and tumor cell survival in Hodgkin lymphoma.
Szydłowski M, Prochorec-Sobieszek M, Szumera-Ciećkiewicz A, Derezińska E, Hoser G, Wasilewska D, Szymańska-Giemza O, Jabłońska E, Białopiotrowicz E, Sewastianik T, Polak A, Czardybon W, Gałęzowski M, Windak R, Zaucha JM, Warzocha K, Brzózka K, Juszczyński P. Szydłowski M, et al. Blood. 2017 Sep 21;130(12):1418-1429. doi: 10.1182/blood-2017-01-760702. Epub 2017 Jul 11. Blood. 2017. PMID: 28698206 - The role of cytokines in classical Hodgkin lymphoma.
Skinnider BF, Mak TW. Skinnider BF, et al. Blood. 2002 Jun 15;99(12):4283-97. doi: 10.1182/blood-2002-01-0099. Blood. 2002. PMID: 12036854 Review. - Galectin-1 serum levels reflect tumor burden and adverse clinical features in classical Hodgkin lymphoma.
Ouyang J, Plütschow A, Pogge von Strandmann E, Reiners KS, Ponader S, Rabinovich GA, Neuberg D, Engert A, Shipp MA. Ouyang J, et al. Blood. 2013 Apr 25;121(17):3431-3. doi: 10.1182/blood-2012-12-474569. Epub 2013 Feb 26. Blood. 2013. PMID: 23444403 - Interaction between host T cells and Reed-Sternberg cells in Hodgkin lymphomas.
Poppema S, van den Berg A. Poppema S, et al. Semin Cancer Biol. 2000 Oct;10(5):345-50. doi: 10.1006/scbi.2000.0327. Semin Cancer Biol. 2000. PMID: 11100882 Review.
Cited by
- Antitumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding.
Dings RP, Miller MC, Nesmelova I, Astorgues-Xerri L, Kumar N, Serova M, Chen X, Raymond E, Hoye TR, Mayo KH. Dings RP, et al. J Med Chem. 2012 Jun 14;55(11):5121-9. doi: 10.1021/jm300014q. Epub 2012 May 30. J Med Chem. 2012. PMID: 22575017 Free PMC article. - Leveraging fluorinated glucosamine action to boost antitumor immunity.
Dimitroff CJ. Dimitroff CJ. Curr Opin Immunol. 2013 Apr;25(2):206-13. doi: 10.1016/j.coi.2012.11.003. Epub 2012 Dec 6. Curr Opin Immunol. 2013. PMID: 23219268 Free PMC article. Review. - [Galectin-1 knockdown inhibits proliferation, migration, invasion and promotes apoptosis of lung adenocarcinoma cells in vitro].
Chen W, Zhu X, Zhou S, Xing F, Tang Z, Li X, Zhang L. Chen W, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2022 Nov 20;42(11):1628-1637. doi: 10.12122/j.issn.1673-4254.2022.11.06. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36504055 Free PMC article. Chinese. - SIRT1 and HSP90α feed-forward circuit safeguards chromosome segregation integrity in diffuse large B cell lymphomas.
Białopiotrowicz-Data E, Noyszewska-Kania M, Jabłońska E, Sewastianik T, Komar D, Dębek S, Garbicz F, Wojtas M, Szydłowski M, Polak A, Górniak P, Juszczyński P. Białopiotrowicz-Data E, et al. Cell Death Dis. 2023 Oct 11;14(10):667. doi: 10.1038/s41419-023-06186-0. Cell Death Dis. 2023. PMID: 37816710 Free PMC article. - Galectins in Intestinal Inflammation: Galectin-1 Expression Delineates Response to Treatment in Celiac Disease Patients.
Sundblad V, Quintar AA, Morosi LG, Niveloni SI, Cabanne A, Smecuol E, Mauriño E, Mariño KV, Bai JC, Maldonado CA, Rabinovich GA. Sundblad V, et al. Front Immunol. 2018 Mar 1;9:379. doi: 10.3389/fimmu.2018.00379. eCollection 2018. Front Immunol. 2018. PMID: 29545799 Free PMC article.
References
- Re D, Kuppers R, Diehl V. J Clin Oncol. 2005;23:6379–6386. - PubMed
- Kuppers R, Schwering I, Brauinger A, Rajewsky K, Hansmann ML. Ann Oncol. 2002;13:11–18. - PubMed
- Schwering I, Brauninger A, Klein U, Jungnickel B, Tinguely M, Diehl V, Hansmann ML, Dalla-Favera R, Rajewsky K, Kuppers R. Blood. 2003;101:1505–1512. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous