An endogenous serine/threonine protein phosphatase inhibitor, G-substrate, reduces vulnerability in models of Parkinson's disease - PubMed (original) (raw)
Comparative Study
An endogenous serine/threonine protein phosphatase inhibitor, G-substrate, reduces vulnerability in models of Parkinson's disease
Chee Yeun Chung et al. J Neurosci. 2007.
Abstract
Relative neuronal vulnerability is a universal yet poorly understood feature of neurodegenerative diseases. In Parkinson's disease, dopaminergic (DA) neurons in the substantia nigra (SN) (A9) are particularly vulnerable, whereas adjacent DA neurons within the ventral tegmental area (A10) are essentially spared. Our previous laser capture microdissection and microarray study (Chung et al., 2005) demonstrated that molecular differences between these DA neurons may underlie their differential vulnerability. Here we show that G-substrate, an endogenous inhibitor of Ser/Thr protein phosphatases, exhibits higher expression in A10 compared with A9 DA neurons in both rodent and human midbrain. Overexpression of G-substrate protected dopaminergic BE(2)-M17 cells against toxins, including 6-OHDA and MG-132 (carbobenzoxy-L-leucyl- L-leucyl-L-leucinal), whereas RNA interference (RNAi)-mediated knockdown of endogenous G-substrate increased their vulnerability to these toxins. G-substrate reduced 6-OHDA-mediated protein phosphatase 2A (PP2A) activation in vitro and increased phosphorylated levels of PP2A targets including Akt, glycogen synthase kinase 3beta, and extracellular signal-regulated kinase 2 but not p38. RNAi to Akt diminished the protective effect of G-substrate against 6-OHDA. In vivo, lentiviral delivery of G-substrate to the rat SN increased baseline levels of phosphorylated Akt and protected A9 DA neurons from 6-OHDA-induced toxicity. These results suggest that inherent differences in the levels of G-substrate contribute to the differential vulnerability of DA neurons and that enhancing G-substrate levels may be a neuroprotective strategy for the vulnerable A9 (SN) DA neurons in Parkinson's disease.
Figures
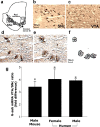
Figure 1.
G-substrate mRNA levels in mouse and human DA neurons. b–f, DA neurons were labeled using the quick TH staining method in mouse, as described previously (Chung et al., 2005), and human midbrain. d–f, DA neurons were then collected using laser capture microdissection. g, Quantitative PCR results demonstrated that A10 DA neurons showed higher levels of G-substrate mRNA than A9 DA neurons in both rodent and human. Data are shown as mRNA ratios of A10 over A9 DA neurons ± SEM (n = 3 for mouse, n = 4 for male human, and n = 3 for female human; *p < 0.05, two-tailed t test). SNc, Substantia nigra pars compacta.
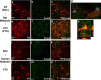
Figure 2.
G-substrate expression in rat and human midbrain DA neurons. TH (a, b, i, l) and G-substrate (b, f, j, m) were stained in rat (a–g) and human (i–n) midbrain. More intense G-substrate staining was observed in A10 DA neurons (e–g, l–n) compared with A9 DA neurons (a–c, i–k). h, Colocalization of G-substrate and TH was confirmed by the _z_-stack confocal image.
Figure 3.
Overexpression of wild-type and mutant (T123A) G-substrate in BE(2)-M17 cells. Lentivirus containing the wild-type and T123A G-substrate gene were transduced to BE(2)-M17 cells with a multiplicity of infection of 15. a, b, Overexpression of the wild-type and T123A G-substrate was confirmed by Western blot (a) and immunocytochemistry (b). c–f, After exposing these cells to 6-OHDA (c, d) or MG-132 (e, f), cell viability was measured using MTS assay (c, e), and cytotoxicity was measured via LDH release (d, f). Overexpression of both the wild-type and T123A G-substrate was protective against 6-OHDA and MG-132 toxicity. There was significantly less protection in T123A G-substrate-expressing cells compared with the wild-type G-substrate-expressing cells. Data are shown as means ± SEM (n = 6–8) and are representatives of three or more experiments with the similar trends (§ p < 0.001, two-way ANOVA; *p < 0.001, not significant, one-way ANOVA, Tukey's test). g, h, Knockdown of the endogenous G-substrate using siRNA: cell viability was measured using the MTS assay, and results are expressed as a percentage of cells exposed to control siRNA without 6-OHDA treatment. The endogenous G-substrate knockdown increased vulnerability of the cells to 6-OHDA toxicity (g) and MG-132 toxicity (h). Data are shown as means ± SEM (n = 6–8) and are representatives of three or more experiments with the similar trends (**p < 0.001, not significant, one-way ANOVA, Tukey's test).
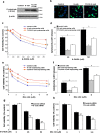
Figure 4.
Changes in phosphorylated G-substrate and PP2A activity after 6-OHDA exposure. a, G-substrate was immunoprecipitated from control and G-substrate-expressing cells, and phosphorylated and total G-substrate levels were measured in the absence or presence of 6-OHDA treatment. G-substrate overexpression caused an increase in phosphorylated G-substrate levels in the basal conditions, which was further augmented by 6-OHDA exposure. To quantify the levels of phospho-G-substrate, optical densities (OD) of the phospho-G-substrate levels in each condition was normalized with basal (no 6-OHDA) phosphorylated G-substrate in control cells. Statistical comparisons were made against no 6-OHDA control cell conditions (n = 3, *p < 0.01, two-tailed t test). b, PP2A activity was measured at various times (t = 0, 1, 2, 5, and 8 h) on BE(2)-M17 cells after 50 μ
m
6-OHDA treatment. Exposure to 6-OHDA induced an increase in PP2A activity at all times except t = 5 h. Data are shown as means ± SEM (n = 3). Statistical comparisons were made against baseline control values at t = 0 h (*p < 0.05, two-tailed t test). c, PP2A activity was measured in control, wild-type, and T123A G-substrate-expressing cells after exposing cells to 50 μ
m
6-OHDA for 2 h. The results are expressed as a percentage of the basal (no 6-OHDA condition) activity of the control cells. Surprisingly, overexpression of the wild-type and T123A G-substrate increased the basal PP2A activity (c). In response to 6-OHDA exposure, the wild-type G-substrate significantly reduced the observed 6-OHDA-induced PP2A activity increase. The degree of reduction in T123A G-substrate-expressing cells was significantly lower than the wild-type-expressing cells. For okadaic acid-treated conditions, 1 n
m
okadaic acid was applied for 30 min before harvesting the cells for PP2A activity measurement. Data are shown as means ± SEM of five independent experiments (**p < 0.005, two-tailed t test). d, Western blot analysis of immunoprecipitated PP2A indicates that the activity changes shown in b were not attributable to alterations of PP2A protein levels.
Figure 5.
Changes in phosphorylation levels of cellular PP2A substrates. Both control and the wild-type G-substrate-expressing cells were exposed to 50 μ
m
6-OHDA for various durations (t = 0, 2, 5, and 10 h). a, c, e, h, The levels of total and phosphorylated form of PP2A substrates were detected by Western blot analysis. b, d, f, g, i, The optical densities (OD) of the individual bands were quantified using NIH Image. Optical densities of phosphorylated epitopes were normalized with those of total epitopes as an internal control. These values were normalized with optical densities at time t = 0 of the control cells. a, b, G-substrate overexpression caused an increase in basal levels of pAkt as well as t = 2, 5, and10 h. c, d, The basal pGSK3β levels were increased in G-substrate-expressing cells. pErk2 levels were significantly increased in G-substrate-expressing cells at t = 10 h (e–g), whereas pEkr1 and pp38 levels were not significantly altered by G-substrate expression. Data are shown as means ± SEM of four to five independent experiments (*p < 0.05, Holm–Sidak post hoc test).
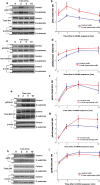
Figure 6.
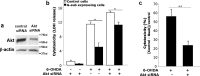
Knockdown of endogenous Akt decreases G-substrate-induced protection against 6-OHDA. a, Western blot analysis confirmed that Akt siRNA application in BE(2)-M17 cells substantially reduced the Akt protein levels. b, Cytotoxicity was measured in the presence or absence of 50 μ
m
6-OHDA treatment after applying control or Akt siRNA to both control and G-substrate-expressing cells. Data are shown as means ± SEM of four independent experiments (*p < 0.001, one-way ANOVA, Tukey's test). These data were used to generate the percentage decrease in cytotoxicity afforded by G-substrate [(control − G-substrate)/control] in the presence of Akt (55.6 ± 8.1%) or with Akt knocked down (23.1 ± 4.9%; c). Data are shown as means ± SEM of four independent experiments (**p < 0.05, two-tailed t test).
Figure 7.
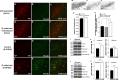
Nigral injection of G-substrate lentivirus protects midbrain dopaminergic neurons in retrograde 6-OHDA lesion model. TH (a, d, g, j) and G-substrate (b, e, h, k) were stained in rat midbrain. a–c, Endogenous G-substrate expression in untransduced midbrain DA neurons. d–f, When lenti-G-substrate was injected directly above nigra, 14–30% of TH-positive cells showed higher levels of G-substrate expression than endogenous levels. Lenti-G-substrate injection (n = 6) did not alter the total number of TH-positive cells compared with the lenti-GFP-injected rat (n = 6) (p) (not significant, two-tailed t test). After 6-OHDA lesion, more TH-positive neurons remained in lenti-G-substrate-injected rats (n = 9) compared with control lentivirus (empty vector, n = 9; or YFP, n = 14) injected nigra (q). Data are shown as means ± SEM (*p < 0.004, **p < 0.001, one-way ANOVA, Tukey's test). j–l, Most of the remaining TH-positive neurons in lenti-G-substrate-injected midbrain were strongly G-substrate-positive r, s, The G-substrate-transduced nigra (n = 10) shows increased levels of pAkt compared with the control (empty vector, n = 10) lentivirus transduced nigra, whereas pGSK3β levels remained unchanged. t, u, Conversely, the striatum of G-substrate-injected rat showed an increase in both pAkt and pGSK3β levels. Data are shown as mean ± SEM [*p < 0.01, **p < 0.001, two-tailed t test, not significant (n.s.)].
Similar articles
- Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression.
Chung CY, Koprich JB, Hallett PJ, Isacson O. Chung CY, et al. Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22474-9. doi: 10.1073/pnas.0912193106. Epub 2009 Dec 10. Proc Natl Acad Sci U S A. 2009. PMID: 20007772 Free PMC article. - Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death.
Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Chen G, et al. FASEB J. 2004 Jul;18(10):1162-4. doi: 10.1096/fj.04-1551fje. Epub 2004 May 7. FASEB J. 2004. PMID: 15132987 - Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection.
Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Chung CY, et al. Hum Mol Genet. 2005 Jul 1;14(13):1709-25. doi: 10.1093/hmg/ddi178. Epub 2005 May 11. Hum Mol Genet. 2005. PMID: 15888489 Free PMC article. - Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.
Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Lujan R, Malenka RC, Striessnig J, Liss B. Dragicevic E, et al. Brain. 2014 Aug;137(Pt 8):2287-302. doi: 10.1093/brain/awu131. Epub 2014 Jun 16. Brain. 2014. PMID: 24934288 Free PMC article. - Molecular Determinants of A9 Dopaminergic Neurons.
Mishra AK, Dixit S, Singh A, Shukla T, Rizvi SI. Mishra AK, et al. Neuromolecular Med. 2025 May 21;27(1):43. doi: 10.1007/s12017-025-08861-1. Neuromolecular Med. 2025. PMID: 40397062 Review.
Cited by
- The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons.
Chung CY, Licznerski P, Alavian KN, Simeone A, Lin Z, Martin E, Vance J, Isacson O. Chung CY, et al. Brain. 2010 Jul;133(Pt 7):2022-31. doi: 10.1093/brain/awq142. Epub 2010 Jun 23. Brain. 2010. PMID: 20573704 Free PMC article. - Neuroprotective Effects of Intranasal IGF-1 against Neonatal Lipopolysaccharide-Induced Neurobehavioral Deficits and Neuronal Inflammation in the Substantia Nigra and Locus Coeruleus of Juvenile Rats.
Tien LT, Lee YJ, Pang Y, Lu S, Lee JW, Tseng CH, Bhatt AJ, Savich RD, Fan LW. Tien LT, et al. Dev Neurosci. 2017;39(6):443-459. doi: 10.1159/000477898. Epub 2017 Aug 5. Dev Neurosci. 2017. PMID: 28787734 Free PMC article. - Molecular heterogeneity of midbrain dopaminergic neurons--Moving toward single cell resolution.
Anderegg A, Poulin JF, Awatramani R. Anderegg A, et al. FEBS Lett. 2015 Dec 21;589(24 Pt A):3714-26. doi: 10.1016/j.febslet.2015.10.022. Epub 2015 Oct 23. FEBS Lett. 2015. PMID: 26505674 Free PMC article. Review. - The importance of molecular histology to study glial influence on neurodegenerative disorders. Focus on recent developed single cell laser microdissection.
Chadi G, Maximino JR, de Oliveira GP. Chadi G, et al. J Mol Histol. 2009 Aug;40(4):241-50. doi: 10.1007/s10735-009-9235-0. Epub 2009 Nov 1. J Mol Histol. 2009. PMID: 19882358 Review. - Nitrated alpha-synuclein induces the loss of dopaminergic neurons in the substantia nigra of rats.
Yu Z, Xu X, Xiang Z, Zhou J, Zhang Z, Hu C, He C. Yu Z, et al. PLoS One. 2010 Apr 8;5(4):e9956. doi: 10.1371/journal.pone.0009956. PLoS One. 2010. PMID: 20386702 Free PMC article.
References
- Alvarado-Kristensson M, Andersson T. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J Biol Chem. 2005;280:6238–6244. - PubMed
- Aswad DW, Greengard P. A specific substrate from rabbit cerebellum for guanosine 3′:5′-monophosphate-dependent protein kinase. I. Purification and characterization. J Biol Chem. 1981;256:3487–3493. - PubMed
- Chatfield K, Eastman A. Inhibitors of protein phosphatases 1 and 2A differentially prevent intrinsic and extrinsic apoptosis pathways. Biochem Biophys Res Commun. 2004;323:1313–1320. - PubMed
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. - PubMed
- Choi WS, Eom DS, Han BS, Kim WK, Han BH, Choi EJ, Oh TH, Markelonis GJ, Cho JW, Oh YJ. Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. J Biol Chem. 2004;279:20451–20460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical