Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials - PubMed (original) (raw)
Review
. 2007 Aug 28;177(5):469-79.
doi: 10.1503/cmaj.070948. Epub 2007 Aug 1.
Affiliations
- PMID: 17671238
- PMCID: PMC1950172
- DOI: 10.1503/cmaj.070948
Review
Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials
Lisa Rambout et al. CMAJ. 2007.
Abstract
Background: Human papillomavirus (HPV) is now known to be a necessary cause of cervical cancer, and prophylactic HPV vaccines aimed at preventing genital warts, precancerous cervical lesions and cervical cancer are now available. To gauge the potential impact on disease burden, we performed a systematic review of the evidence from randomized controlled trials.
Methods: We conducted a systematic search of the literature to identify all randomized controlled trials of prophylactic HPV vaccination. Reports in 5 electronic databases covering 1950 to June 2007 (MEDLINE, MEDLINE in process, EMBASE, the Cochrane Central Registry of Controlled Trials and the Cochrane Library), bibliographies of all included studies and of narrative reviews (2006-2007), clinical trial registries, Google Scholar, public health announcements, selected conference proceedings (2004-2007) and manufacturers' information on unpublished data or ongoing trials were screened against predefined eligibility criteria by 2 independent reviewers. Vaccines had to contain coverage against at least 1 oncogenic HPV strain. The primary outcome of interest was the frequency of high-grade cervical lesions (high-grade squamous intraepithelial lesion, or grade 2 or 3 cervical intraepithelial neoplasia). The secondary outcomes were persistent HPV infection, low-grade cervical lesions (low-grade squamous intraepithelial lesion or grade 1 cervical intraepithelial neoplasia), external genital lesions, adverse events and death. Meta-analysis of the data was done in all cases where adequate clinical and methodological homogeneity existed.
Results: Of 456 screened reports, 9 were included in the review (6 were reports of randomized controlled trials and 3 were follow-up reports of initial trials). Findings from the meta-analysis showed that prophylactic HPV vaccination was associated with a reduction in the frequency of high-grade cervical lesions caused by vaccine-type HPV strains compared with control groups: Peto odds ratio 0.14 (95% confidence interval [CI] 0.09-0.21) from combined per-protocol analyses, and 0.52 (95% CI 0.43-0.63) from modified intention-to-treat analyses. Vaccination was also highly efficacious in preventing other HPV-related infection and disease outcomes, including persistent HPV infection, low-grade lesions and genital warts. The majority of adverse events were minor. The incidence of serious adverse events and death were balanced between the vaccine and control groups.
Interpretation: Among women aged 15-25 years not previously infected with vaccine-type HPV strains, prophylactic HPV vaccination appears to be highly efficacious in preventing HPV infection and precancerous cervical disease. Long-term follow-up is needed to substantiate reductions in cervical cancer incidence and mortality.
Figures
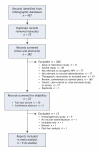
Figure 1: Selection of studies for meta-analysis.
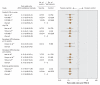
Figure 2: Per-protocol meta-analysis of clinically important outcomes in selected studies of prophylactic vaccination against human papillomavirus (HPV)-related infection and disease. (Per-protocol analyses included study participants who were seronegative and DNA negative for relevent HPV types at enrolment and who received all 3 doses of vaccine.) CIN = cervical intraepithelial neoplasia.
Figure 3: Modified intention-to-treat meta-analysis of clinically important outcomes in selected studies of prophylactic vaccination against human papillomavirus (HPV)-related infection and disease. (Modified intention-to-treat analyses included study participants who received at least 1 dose of vaccine and who either were negative for relevant HPV types at enrolment [Harper, Villa, PATRICIA] or were randomly assigned to study group irrespective of their baseline HPV status [Mao, FUTURE I, FUTURE II].) CIN = cervical intraepithelial neoplasia.
Figure 4: Meta-analysis of serious adverse events and death in selected studies of prophylactic vaccination against HPV-related infection and disease.
Comment in
- Vaccination against human papillomavirus.
Brophy JM. Brophy JM. CMAJ. 2007 Dec 4;177(12):1525-6; author reply 1527-8. doi: 10.1503/cmaj.1070128. CMAJ. 2007. PMID: 18056602 Free PMC article. No abstract available. - Review: vaccines against human papillomavirus prevent cervical cancer precursors in young women.
Muresan J. Muresan J. Evid Based Nurs. 2008 Jan;11(1):11. doi: 10.1136/ebn.11.1.11. Evid Based Nurs. 2008. PMID: 18192516 No abstract available.
Similar articles
- Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.
Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Arbyn M, et al. Cochrane Database Syst Rev. 2018 May 9;5(5):CD009069. doi: 10.1002/14651858.CD009069.pub3. Cochrane Database Syst Rev. 2018. PMID: 29740819 Free PMC article. Review. - Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males.
Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros-Balta AX, Low N, Henschke N. Bergman H, et al. Cochrane Database Syst Rev. 2019 Nov 22;2019(11):CD013479. doi: 10.1002/14651858.CD013479. Cochrane Database Syst Rev. 2019. PMID: 31755549 Free PMC article. - Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials.
Lehtinen M, Lagheden C, Luostarinen T, Eriksson T, Apter D, Harjula K, Kuortti M, Natunen K, Palmroth J, Petäjä T, Pukkala E, Siitari-Mattila M, Struyf F, Nieminen P, Paavonen J, Dubin G, Dillner J. Lehtinen M, et al. BMJ Open. 2017 Aug 18;7(8):e015867. doi: 10.1136/bmjopen-2017-015867. BMJ Open. 2017. PMID: 28821519 Free PMC article. Clinical Trial. - Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women.
Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia PJ, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Huh WK, Joura EA, Kurman RJ, Majewski S, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan JT, Lupinacci LC, Giacoletti KE, Sings HL, James MK, Hesley TM, Barr E, Haupt RM. Muñoz N, et al. J Natl Cancer Inst. 2010 Mar 3;102(5):325-39. doi: 10.1093/jnci/djp534. Epub 2010 Feb 5. J Natl Cancer Inst. 2010. PMID: 20139221 Clinical Trial. - Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis.
Lu B, Kumar A, Castellsagué X, Giuliano AR. Lu B, et al. BMC Infect Dis. 2011 Jan 12;11:13. doi: 10.1186/1471-2334-11-13. BMC Infect Dis. 2011. PMID: 21226933 Free PMC article. Review.
Cited by
- Applying a gender lens on human papillomavirus infection: cervical cancer screening, HPV DNA testing, and HPV vaccination.
Branković I, Verdonk P, Klinge I. Branković I, et al. Int J Equity Health. 2013 Feb 8;12:14. doi: 10.1186/1475-9276-12-14. Int J Equity Health. 2013. PMID: 23394214 Free PMC article. Review. - Circumcision for all: the pro side.
Houle AM. Houle AM. Can Urol Assoc J. 2007 Nov;1(4):398-400. Can Urol Assoc J. 2007. PMID: 18542826 Free PMC article. No abstract available. - Two hypotheses of dense breasts and viral infection for explaining incidence of breast cancer by age group in Korean women.
Bae JM. Bae JM. Epidemiol Health. 2014 Sep 26;36:e2014020. doi: 10.4178/epih/e2014020. eCollection 2014. Epidemiol Health. 2014. PMID: 25266421 Free PMC article. - Strategies for evaluating the assumptions of the regression discontinuity design: a case study using a human papillomavirus vaccination programme.
Smith LM, Lévesque LE, Kaufman JS, Strumpf EC. Smith LM, et al. Int J Epidemiol. 2017 Jun 1;46(3):939-949. doi: 10.1093/ije/dyw195. Int J Epidemiol. 2017. PMID: 28338752 Free PMC article. - Warts (genital).
W Buck H Jr. W Buck H Jr. BMJ Clin Evid. 2010 Aug 13;2010:1602. BMJ Clin Evid. 2010. PMID: 21418685 Free PMC article. Review.
References
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-27. - PubMed
- Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24(Suppl 3):S26-34. - PubMed
- Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-26. - PubMed
- Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369: 1861-8. - PubMed
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007;369:1693-702. - PubMed