The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer - PubMed (original) (raw)
Review
The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer
Kathryn M Taylor et al. Mol Med. 2007 Jul-Aug.
Abstract
Zinc transporter LIV-1 (SLC39A6) is estrogen regulated and present in increased amounts in estrogen receptor-positive breast cancer as well as in tumors that spread to the lymph nodes. The LIV-1 subfamily of ZIP zinc transporters consists of nine human sequences that share considerable homology across transmembrane domains. Many of these sequences have been shown to transport zinc and/or other ions across cell membranes. Increasingly, studies have implicated members of the LIV-1 transporter subfamily in a variety of diseases. We review these studies and report our own investigations of the role in breast cancer of the nine LIV-1 zinc transporters. We have documented the response of these transporters to estrogen and antiestrogens, and also their presence in our models of resistance to antiestrogens. Resistance to antiestrogen drugs such as tamoxifen and fulvestrant often occurs in advanced breast cancer. In these models we observed differential expression of individual LIV-1 family members, which may be related to their observed variable tissue expression. We were unable detect ZIP4, which is known to be expressed in the intestine. HKE4/SLC39A7 had elevated expression in both antiestrogen-resistant cell lines, and ZIP8 had elevated expression in fulvestrant-resistant cells. In addition, we investigated the expression of the nine LIV-1 family members in a clinical breast cancer series. Although a number of different LIV-1 family members showed some association with growth factor receptors, LIV-1 was solely associated with estrogen receptor and a variety of growth factors commonly associated with clinical breast cancer. HKE4, however, did show an association with the marker of cell proliferation Ki67 the spread of breast cancer to lymph nodes.
Figures
Figure 1
Phylogenetic tree and alignment of the human members of the ZIP superfamily of zinc transporters. (A) This phylogenetic tree was drawn using ClustalW and treeview software. (B) This alignment demonstrating the highly conserved motif in TM V for the LIV-1 family was performed using ClustalW and shaded using Boxshade software. Black shading represents at least 50% identity, gray shading represents at least 50% complementary residues. (C) This alignment demonstrating the CPALLY motif directly upstream of TM I was performed using ClustalW and shaded using Boxshade software. Black shading represents at least 50% identity, gray shading represents at least 50% complementary residues.
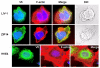
Figure 2
Cellular location of three human LIV-1 family members in MCF-7 cells. MCF-7 cells expressing recombinant HKE4, LIV-1, or ZIP14 were imaged using a mouse anti-V5 antibody (Invitrogen) conjugated to Alexa Fluor 488 (green) and assembled onto slides using Vectorshield with DAPI (Vector Laboratories). All cells were incubated with Texas red phalloidin to stain F-Actin filaments red. Coverslips were viewed on a Leica RPE automatic microscope using a 63x oil immersion lens. The fluorescent superimposed images were acquired using a multiple bandpass filter set appropriate for DAPI, fluorescein, and Texas Red as well as bright field for differential interference contrast imaging.
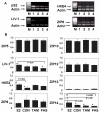
Figure 3
Comparison of the effect of antihormones on the RNA expression of the nine human LIV-1 family members. MCF-7 cells were treated with 4-hydroxytamoxifen (TAM), fulvestrant (FAS), or oestradiol (E2) for seven days in serum growth factor–free DCCM medium before total RNA was extracted, reverse transcribed to cDNA, and PCR performed for individual ZIPs. All data were normalized to individual β-actin levels. The upper panel shows representative agarose gels of pS2, LIV-1, HKE4, and ZIP8 in MCF-7 treated with oestradiol (lane 1), untreated (lane 2), treated with Tamoxifen (lane 3), or treated with Fulvestrant (lane 4). M represents size markers. Lower panel shows mean ± SEM densitometric values of 8 genes, comparing their expression levels in MCF-7 cells untreated (CON), or treated with oestradiol (E2), Tamoxifen (TAM), or Fulvestrant (FAS). Statistically significant results are indicated with asterisks, and the relevant P value is given. ZIP4 was undetectable.
Figure 4
Comparison of RNA expression of the nine human LIV-1 family members in anti-hormone-resistant MCF-7 cells. The expression of different ZIPs in tamoxifen (TamR)- and fulvestrant (FasR)-resistant MCF-7 cells was compared with wild-type MCF-7 cells. RNA was extracted and reverse transcribed to cDNA. The upper panel shows representative gels of pS2, LIV-1, HKE4, and ZIP8 in MCF-7 (lane 1), TamR (lane 2) and FasR (lane 3) cell lines. M represents size markers. Lower panel shows mean ± SEM densitometric values of 8 genes comparing their expression levels in MCF-7, TamR, and FasR cells. Statistically significant results are indicated with asterisks, and the relevant P value is given. ZIP4 was undetectable.
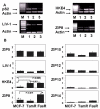
Figure 5
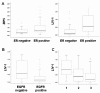
Comparison of the expression of LIV-1 family members in a series of samples from breast cancer patients. (A) representative results from 10 patient tumor samples amplified with gene specific primers for ZIP5, HKE4, ZIP10, LIV-1, ZIP8, ZIP14, and β-actin. (B) distribution of the densitometric values for ZIP5, HKE4, ZIP10, LIV-1, ZIP8, and ZIP14 in the patient samples, with no correction for the number of PCR cycles.
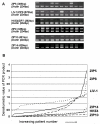
Figure 6
Comparison of the expression of LIV-1 and/or ZIP5 with ER, EGFR and grade in a series of breast cancer samples. (A) shows the positive correlation of both LIV-1 and ZIP5 with estrogen receptor (ER). (B) shows the reverse relationship of LIV-1 with EGFR. (C) shows the decreasing levels of LIV-1 with worsening grade.
Similar articles
- Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters.
Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Taylor KM, et al. Biochem J. 2003 Oct 1;375(Pt 1):51-9. doi: 10.1042/BJ20030478. Biochem J. 2003. PMID: 12839489 Free PMC article. - Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients.
Kasper G, Weiser AA, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castaños-Vélez E, Lehmann K. Kasper G, et al. Int J Cancer. 2005 Dec 20;117(6):961-73. doi: 10.1002/ijc.21235. Int J Cancer. 2005. PMID: 15986450 - The LZT proteins; the LIV-1 subfamily of zinc transporters.
Taylor KM, Nicholson RI. Taylor KM, et al. Biochim Biophys Acta. 2003 Apr 1;1611(1-2):16-30. doi: 10.1016/s0005-2736(03)00048-8. Biochim Biophys Acta. 2003. PMID: 12659941 Review. - A distinct role in breast cancer for two LIV-1 family zinc transporters.
Taylor KM. Taylor KM. Biochem Soc Trans. 2008 Dec;36(Pt 6):1247-51. doi: 10.1042/BST0361247. Biochem Soc Trans. 2008. PMID: 19021534 - Zinc and cancer: implications for LIV-1 in breast cancer.
Grattan BJ, Freake HC. Grattan BJ, et al. Nutrients. 2012 Jul;4(7):648-75. doi: 10.3390/nu4070648. Epub 2012 Jul 4. Nutrients. 2012. PMID: 22852056 Free PMC article. Review.
Cited by
- The SLC39 family of zinc transporters.
Jeong J, Eide DJ. Jeong J, et al. Mol Aspects Med. 2013 Apr-Jun;34(2-3):612-9. doi: 10.1016/j.mam.2012.05.011. Mol Aspects Med. 2013. PMID: 23506894 Free PMC article. Review. - Zinc transporters, mechanisms of action and therapeutic utility: implications for type 2 diabetes mellitus.
Myers SA, Nield A, Myers M. Myers SA, et al. J Nutr Metab. 2012;2012:173712. doi: 10.1155/2012/173712. Epub 2012 Dec 12. J Nutr Metab. 2012. PMID: 23304467 Free PMC article. - Characterization of Zinc Influx Transporters (ZIPs) in Pancreatic β Cells: ROLES IN REGULATING CYTOSOLIC ZINC HOMEOSTASIS AND INSULIN SECRETION.
Liu Y, Batchuluun B, Ho L, Zhu D, Prentice KJ, Bhattacharjee A, Zhang M, Pourasgari F, Hardy AB, Taylor KM, Gaisano H, Dai FF, Wheeler MB. Liu Y, et al. J Biol Chem. 2015 Jul 24;290(30):18757-69. doi: 10.1074/jbc.M115.640524. Epub 2015 May 12. J Biol Chem. 2015. PMID: 25969539 Free PMC article. - Physiologic implications of metal-ion transport by ZIP14 and ZIP8.
Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD. Jenkitkasemwong S, et al. Biometals. 2012 Aug;25(4):643-55. doi: 10.1007/s10534-012-9526-x. Epub 2012 Feb 9. Biometals. 2012. PMID: 22318508 Free PMC article. Review. - A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3).
Hogstrand C, Kille P, Ackland ML, Hiscox S, Taylor KM. Hogstrand C, et al. Biochem J. 2013 Oct 15;455(2):229-37. doi: 10.1042/BJ20130483. Biochem J. 2013. PMID: 23919497 Free PMC article.
References
- Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–59. - PubMed
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological Rev. 1993;73:79–118. - PubMed
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in Caspase activation and apoptotic cell death. Biometals. 2001;14:315–30. - PubMed
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–6. - PubMed
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical