On the formation of gamma-coherent cell assemblies by oriens lacunosum-moleculare interneurons in the hippocampus - PubMed (original) (raw)
On the formation of gamma-coherent cell assemblies by oriens lacunosum-moleculare interneurons in the hippocampus
Adriano B L Tort et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Gamma frequency (30-80 Hz) network oscillations have been observed in the hippocampus during several behavioral paradigms in which they are often modulated by a theta frequency (4-12 Hz) oscillation. Interneurons of the hippocampus have been shown to be crucially involved in rhythms generation, and several subtypes with distinct anatomy and physiology have been described. In particular, the oriens lacunosum-moleculare (O-LM) interneurons were shown to synapse on distal apical dendrites of pyramidal cells and to spike preferentially at theta frequency, even in the presence of gamma-field oscillations. O-LM cells have also recently been shown to present higher axonal ramification in the longitudinal axis of the hippocampus. By using a hippocampal network model composed of pyramidal cells and two types of interneurons (O-LM and basket cells), we show here that the O-LM interneurons lead to gamma coherence between anatomically distinct cell modules. We thus propose that this could be a mechanism for coupling longitudinally distant cells excited by entorhinal cortex inputs into gamma-coherent assemblies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
O-LM cells posses higher axonal projections in the longitudinal direction of the CA3 area. Neurolucida reconstructed biocytin-filled O-LM cell in area CA3 from transverse (Left) and longitudinal slices (Right). The soma and dendrites are drawn in red, whereas the axon is in green. The horizontal dendritic branches were restricted to the stratum oriens. The axons crossed the pyramidal cell layer and extensively innervated the stratum lacunosum-moleculare of the area CA3. Note the much longer axonal ramification pattern in stratum lacunosum-moleculare of the longitudinal slice than of the transverse slice. Hippocampal layers are depicted schematically. CA3, CA3 area; str. or., stratum oriens; str. pyr., stratum pyramidale; str. rad., stratum radiatum; str. l.-m., stratum lacunosum-moleculare.
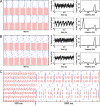
Fig. 2.
O-LM cells coordinate multiple cell assemblies. (A) Network scheme. Within each module, O-LM (O) cell population inhibits the distal apical dendrites of pyramidal (E) cell population, basket (I) cell population inhibits E cell at the soma and also inhibits itself and O cell population, and E cell excites both O and I cells. Connections among modules are made through O–E synapses on distal apical dendrites. (B) Representative spike rastergram of a network consisting of four modules (labeled 1 to 4 on the y axis). Each module consisted of 40 E (black), 10 O (blue), and 10 I cells (red). For clarity, however, just half the number of cells is shown. During the first 500 ms, all E cells received drive currents uniformly distributed from 217–236 pA. Note that E cells spike sparsely and randomly (6.72 ± 2.76 Hz) in this regime and that O–E synapses are able to induce synchrony among modules. For the second 500 ms of simulation, 12 E cells inside each module were activated with higher drive currents. E cells of modules 1 and 2 received random drive from 330–349 pA, whereas activated E cells in modules 3 and 4 received drive from 820–849 pA. Note the suppression of the less active E cells inside each module as well as the formation of two distinct gamma assemblies induced by O–E synapses. (C) Normalized phase difference histograms obtained for the parameter regime shown in the first 500 ms of B, showing synchrony among all modules. (D) Same as in C, but for the regime shown in the second 500 ms of B. Note the loss of synchrony between modules with distinct levels of excitation. (E) Power spectra of the model LFPs of modules 1 and 4 after the activation of subsets of E cells showing theta and (distinct) gamma peaks. (F) Coherence analysis between modules of similar and distinct activated E cell drives. Note the loss of gamma coherence between modules of distinct drive along with no change in theta coherence. C–F were obtained by analyzing 10 s of simulation. E cell drive was applied at the distal apical dendritic compartment. Other parameters are presented in
SI Table 1
. The same color convention for the cells will be used in the other rastergrams.
Fig. 3.
O–E synapses lead to gamma synchrony between modules even when the O cells spike asynchronously. (A) Representative spike rastergram (Left) of two connected modules (1 E, 25 O, and 25 I cells each). Model LFPs of the bottom module (Center; at Soma and Adend2 compartments; see
SI Appendix
) and the corresponding power spectrum analysis (Right) are shown. These graphs represent the most common behavior of this network: E and I cells of both modules spike synchronously at gamma frequency and the O cells tend to spike at theta clusters, which are often synchronous between modules (_G_OE between modules = 8 mS). (B) Same as in A, showing, however, a case in which the O cells do not display clear spikes at theta clusters (especially in the Lower module). Note the reduction of the peak theta power compared with the case shown in A. However, even in these cases of O cell asynchrony, O–E synapses always lead to gamma synchrony between modules. (C) Representative rastergrams of a network composed of eight modules (1 E, 15 I, and 15 O cells each). O–E synapses allow the coexistence of synchronization of two modules of high drive (848 pA; the two excited Upper modules) and of two modules of low drive (424 pA; the two excited Lower modules), whereas four nonexcited modules remain asynchronous (GOE among modules = 5 mS). The arrow indicates the time synapses were turned on. Note that in this example both gamma assemblies present the same theta rhythm. Other parameters are presented in
SI Table 2
.
Fig. 4.
Influence of delay and synaptic strength on O–E-induced gamma synchrony. (A) (Left) Color histograms of gamma phase difference of two modules for distinct values of _G_OE between modules (expressed as percent of _G_OE within a module). (Right) Values for the corresponding phase lock index (PLI). (B) Gamma synchrony induced by O–E synapses between two modules persists with delay values compatible with the anatomy. (Left) Color histograms of gamma phase difference between two modules are plotted for distinct delay times (_G_OE between modules = 60% of _G_OE within). (Right) Values for the corresponding PLI. Note the tendency of antiphase for delay times near half gamma period. For all these simulations, each module was composed of one E, five O, and five I cells. Other parameters values are the same as in Fig. 3.
Fig. 5.
PING is required for O–E induced gamma synchrony between modules. (A) Color histograms of gamma phase difference between two connected modules for distinct values of E cell drive. Note that a minimal level of E drive is required for synchrony. (B) Plots of E and I cells frequencies (left vertical scale) and the phase lock index (PLI) (right vertical scale) for distinct values of E cell drive. (Inset) O cell frequency (left vertical scale) and the ratio of I cell frequency to O cell frequency (right vertical scale) as a function of E cell drive. Note that gamma synchrony between modules emerges when E cell frequency reaches I cells frequency, which is when the E/I subnetwork starts exhibiting PING. Notice also that both gamma (I cell) and theta (O cell) frequencies covary with E drive. Error bars represent SD. Each module consisted of one E, five I, and five O cells. Other parameters values are the same as in Fig. 3.
Similar articles
- Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro.
Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Gloveli T, et al. J Physiol. 2005 Jan 1;562(Pt 1):131-47. doi: 10.1113/jphysiol.2004.073007. Epub 2004 Oct 14. J Physiol. 2005. PMID: 15486016 Free PMC article. - Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency.
Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Goldin M, et al. J Neurosci. 2007 Sep 5;27(36):9560-72. doi: 10.1523/JNEUROSCI.1237-07.2007. J Neurosci. 2007. PMID: 17804617 Free PMC article. - Spike timing of lacunosom-moleculare targeting interneurons and CA3 pyramidal cells during high-frequency network oscillations in vitro.
Spampanato J, Mody I. Spampanato J, et al. J Neurophysiol. 2007 Jul;98(1):96-104. doi: 10.1152/jn.00188.2007. Epub 2007 May 2. J Neurophysiol. 2007. PMID: 17475718 - Alterations of perisomatic GABA synapses on hippocampal CA1 inhibitory interneurons and pyramidal cells in the kainate model of epilepsy.
Morin F, Beaulieu C, Lacaille JC. Morin F, et al. Neuroscience. 1999;93(2):457-67. doi: 10.1016/s0306-4522(99)00199-2. Neuroscience. 1999. PMID: 10465428 - Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus.
Müller C, Remy S. Müller C, et al. Front Synaptic Neurosci. 2014 Sep 30;6:23. doi: 10.3389/fnsyn.2014.00023. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25324774 Free PMC article. Review.
Cited by
- Hippocampal CA1 Ripples as Inhibitory Transients.
Malerba P, Krishnan GP, Fellous JM, Bazhenov M. Malerba P, et al. PLoS Comput Biol. 2016 Apr 19;12(4):e1004880. doi: 10.1371/journal.pcbi.1004880. eCollection 2016 Apr. PLoS Comput Biol. 2016. PMID: 27093059 Free PMC article. - Modeling fast and slow gamma oscillations with interneurons of different subtype.
Keeley S, Fenton AA, Rinzel J. Keeley S, et al. J Neurophysiol. 2017 Mar 1;117(3):950-965. doi: 10.1152/jn.00490.2016. Epub 2016 Dec 7. J Neurophysiol. 2017. PMID: 27927782 Free PMC article. - Spatiotemporal characteristics and pharmacological modulation of multiple gamma oscillations in the CA1 region of the hippocampus.
Balakrishnan S, Pearce RA. Balakrishnan S, et al. Front Neural Circuits. 2015 Jan 12;8:150. doi: 10.3389/fncir.2014.00150. eCollection 2014. Front Neural Circuits. 2015. PMID: 25628540 Free PMC article. - Cell-Type Specific Inhibition Controls the High-Frequency Oscillations in the Medial Entorhinal Cortex.
Gurgenidze S, Bäuerle P, Schmitz D, Vida I, Gloveli T, Dugladze T. Gurgenidze S, et al. Int J Mol Sci. 2022 Nov 15;23(22):14087. doi: 10.3390/ijms232214087. Int J Mol Sci. 2022. PMID: 36430563 Free PMC article. - Effects of Several Classes of Voltage-Gated Ion Channel Conductances on Gamma and Theta Oscillations in a Hippocampal Microcircuit Model.
Olteanu C, Habibollahi F, French C. Olteanu C, et al. Front Comput Neurosci. 2021 Apr 1;15:630271. doi: 10.3389/fncom.2021.630271. eCollection 2021. Front Comput Neurosci. 2021. PMID: 33867962 Free PMC article.
References
- Engel AK, Fries P, Singer W. Nat Rev Neurosci. 2001;2:704–716. - PubMed
- Singer W. Annu Rev Physiol. 1993;55:349–374. - PubMed
- Gray CM. J Comput Neurosci. 1994;1:11–38. - PubMed
- Fries P, Reynolds JH, Rorie AE, Desimone R. Science. 2001;291:1560–1563. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases