A persistent RNA.DNA hybrid formed by transcription of the Friedreich ataxia triplet repeat in live bacteria, and by T7 RNAP in vitro - PubMed (original) (raw)
A persistent RNA.DNA hybrid formed by transcription of the Friedreich ataxia triplet repeat in live bacteria, and by T7 RNAP in vitro
Ed Grabczyk et al. Nucleic Acids Res. 2007.
Abstract
Expansion of an unstable GAA.TTC repeat in the first intron of the FXN gene causes Friedreich ataxia by reducing frataxin expression. Deficiency of frataxin, an essential mitochondrial protein, leads to progressive neurodegeneration and cardiomyopathy. The degree of frataxin reduction correlates with GAA.TTC tract length, but the mechanism of reduction remains controversial. Here we show that transcription causes extensive RNA.DNA hybrid formation on GAA.TTC templates in bacteria as well as in defined transcription reactions using T7 RNA polymerase in vitro. RNA.DNA hybrids can also form to a lesser extent on smaller, so-called 'pre-mutation' size GAA.TTC repeats, that do not cause disease, but are prone to expansion. During in vitro transcription of longer repeats, T7 RNA polymerase arrests in the promoter distal end of the GAA.TTC tract and an extensive RNA.DNA hybrid is tightly linked to this arrest. RNA.DNA hybrid formation appears to be an intrinsic property of transcription through long GAA.TTC tracts. RNA.DNA hybrids have a potential role in GAA.TTC tract instability and in the mechanism underlying reduced frataxin mRNA levels in Friedreich Ataxia.
Figures
Figure 1.
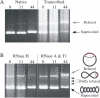
Transcription through a long GAA·TTC tract results in formation of an RNA·DNA hybrid. (A) The native gel mobility of supercoiled templates carrying 0, 11 or 44 GAA·TTC triplets is shown in the first three lanes and after transcription by T7 RNAP in the second three lanes. The RNA product partially obscures the templates. Gel mobilities of relaxed plasmids (gray arrowhead) and supercoiled plasmids (black arrowhead) are indicated. (B) Treatment with RNase H after transcription (first three lanes) returns the (GAA·TTC)44 template to control mobility. Treatment with the single-strand-specific RNases A and T1 (last three lanes) reveals conformers of the (GAA·TTC)44 template (small arrows) with mobilities approaching that of a fully relaxed template (gray arrowhead). The degree of relaxation reflects the length of the RNA·DNA hybrid, which unwinds negative supercoils as indicated in the schematic to the right of the arrows. In contrast, templates with 0 or 11 triplets retain the mobility of untranscribed controls, regardless of treatment.
Figure 2.
Persistent RNA·DNA hybrids form during transcription of GAA·TTC repeats in bacteria. The gel pictured shows the mobility of templates isolated from bacteria in which transcription was repressed by glucose (first 4 lanes) or induced by arabinose (last 4 lanes). The plasmids were treated after isolation with the single-strand-specific RNases A and T1. Some aliquots were additionally treated with RNase H as indicated. The transcribed templates with 88 GAA·TTC triplets show a fully or partially relaxed mobility when treated only with single-strand-specific RNases (next to last lane). Treating an aliquot of the same sample with RNase H returns the bulk of the plasmid to supercoiled mobility in the last lane.
Figure 3.
The RNA·DNA hybrid extends to the promoter proximal end of the repeat tract. Pairs of transcription reactions end-labeled by including gamma 32P-GTP, were done in the absence (−) or presence (+) of RNase H to map the 5′ end of the RNA hybrid. Lanes 1–6 contain the end-labeled products derived from supercoiled templates with 0 (lanes 1 and 2), 11 (lanes 3 and 4) or 44 (lanes 5 and 6) GAA·TTC triplets. Lanes 7 and 8 contain the end-labeled products derived from a linear (GAA·TTC)44 template. The most common end-labeled fragments generated by RNase H were at or near the start of the GAA tract in the templates with 44 triplets (lanes 6 and 12). Lane 13 contains a ‘G ladder’ from a partial RNase T1 digest of end-labeled (GAA)44 transcript. The 3′ end of the (GAA)44 tract at base 187 is indicated by a thin arrow, the first G of the triplet repeat sequence, at base 55 in the transcript 5′ end is indicated by a thick arrow near the bottom of the gel. Full-length transcripts are not resolved on this gel (but see Figure 1).
Figure 4.
RNA·DNA hybrids are associated with transcription arrest on TTC templates in vitro. Pairs of transcription reactions were done in the absence (−) or presence (+) of RNase H on supercoiled templates containing the triplet repeat sequences (CTG·CAG)88 (lanes 1 and 2), (GAA·TTC)88 (lanes 3 and 4) and (TTC·GAA)88 (lanes 5 and 6). The gel image shows the products of those reactions. To the right of the gel image the length in bases of select DNA size markers are indicated. At the far right of the figure is a scan of lanes 3 (dark line) and 4 (gray line) which highlights the RNase H mediated shift in truncation points from promoter distal (arrowhead) to promoter proximal within the GAA repeat. All the templates contain the sequence for a self-cleaving ribozyme that cuts the transcript 270 bases 3′ to the end of the repeat tract producing a full-length transcript of 590 bases. Transcription from the phage T7 promoter started 55 bases 5′ to the GAA·TTC insert. Transcripts were end-labeled by including gamma 32P-GTP in the reaction.
Figure 5.
A class of transcripts cleaved by RNase H can extend beyond the GAA·TTC repeat. (A) Primer extension provides high resolution mapping of the 3′ limit of the RNA·DNA hybrid within the transcript. The supercoiled (GAA·TTC)88 template used in Figure 4 was transcribed in the presence or absence of RNase H to provide RNA for reverse transcriptase primer extension of an end-labeled 30 base oligonucleotide that annealed from 72 to 42 bases beyond the 3′ end of the repeat in the transcript. Full-length extension to the 5′ end of the transcript yields a 390 base product in both samples. The major RNase H cleavage endpoints are clustered within a couple of triplets from the promoter distal end of the repeat sequence (arrow). (B) Experimental design and interpretation.
Figure 6.
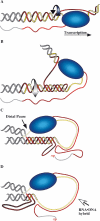
Transcription-coupled RNA·DNA hybrid formation in a GAA·TTC repeat. Model for transient transcription-dependent triplex formation leading to an RNA polymerase pause and RNA·DNA hybrid formation. The purine (GAA or R) strand of the repeat is red, the pyrimidine (TTC or Y) strand is yellow and the flanking DNA is gray. (A) A standing wave of negative supercoiling follows RNA polymerase. At the transcription bubble, the non-template (GAA) strand is available to fold back in an R·R·Y interaction; the template strand is covered by RNA polymerase. (B) Rotation of the helix (curved arrow) as it winds in the third strand relaxes the negative supercoils caused by transcription and leaves a length of the template single-stranded. (C) RNAP is impeded at the distal template–triplex junction and the nascent transcript can anneal to the single-stranded stretch of template. (D) The RNA·DNA hybrid displaces the much less stable triplex structure. Structures of this type can account for the data generated by the 32P-end-labeled transcripts shown in lanes 3 and 4 of Figure 4.
Similar articles
- The GAA*TTC triplet repeat expanded in Friedreich's ataxia impedes transcription elongation by T7 RNA polymerase in a length and supercoil dependent manner.
Grabczyk E, Usdin K. Grabczyk E, et al. Nucleic Acids Res. 2000 Jul 15;28(14):2815-22. doi: 10.1093/nar/28.14.2815. Nucleic Acids Res. 2000. PMID: 10908340 Free PMC article. - DNA sequence-specific polyamides alleviate transcription inhibition associated with long GAA.TTC repeats in Friedreich's ataxia.
Burnett R, Melander C, Puckett JW, Son LS, Wells RD, Dervan PB, Gottesfeld JM. Burnett R, et al. Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11497-502. doi: 10.1073/pnas.0604939103. Epub 2006 Jul 20. Proc Natl Acad Sci U S A. 2006. PMID: 16857735 Free PMC article. - Inhibitory effects of expanded GAA.TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo.
Ohshima K, Montermini L, Wells RD, Pandolfo M. Ohshima K, et al. J Biol Chem. 1998 Jun 5;273(23):14588-95. doi: 10.1074/jbc.273.23.14588. J Biol Chem. 1998. PMID: 9603975 - Beyond loss of frataxin: the complex molecular pathology of Friedreich ataxia.
Evans-Galea MV, Lockhart PJ, Galea CA, Hannan AJ, Delatycki MB. Evans-Galea MV, et al. Discov Med. 2014 Jan;17(91):25-35. Discov Med. 2014. PMID: 24411698 Review. - Gene-based approaches toward Friedreich ataxia therapeutics.
Hebert MD, Whittom AA. Hebert MD, et al. Cell Mol Life Sci. 2007 Dec;64(23):3034-43. doi: 10.1007/s00018-007-7293-6. Cell Mol Life Sci. 2007. PMID: 17828464 Free PMC article. Review.
Cited by
- Oral administration of the pimelic diphenylamide HDAC inhibitor HDACi 4b is unsuitable for chronic inhibition of HDAC activity in the CNS in vivo.
Beconi M, Aziz O, Matthews K, Moumné L, O'Connell C, Yates D, Clifton S, Pett H, Vann J, Crowley L, Haughan AF, Smith DL, Woodman B, Bates GP, Brookfield F, Bürli RW, McAllister G, Dominguez C, Munoz-Sanjuan I, Beaumont V. Beconi M, et al. PLoS One. 2012;7(9):e44498. doi: 10.1371/journal.pone.0044498. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973455 Free PMC article. - Targeted Oligonucleotides for Treating Neurodegenerative Tandem Repeat Diseases.
Zain R, Smith CIE. Zain R, et al. Neurotherapeutics. 2019 Apr;16(2):248-262. doi: 10.1007/s13311-019-00712-9. Neurotherapeutics. 2019. PMID: 31098852 Free PMC article. Review. - From R-Loops to G-Quadruplexes: Emerging New Threats for the Replication Fork.
Maffia A, Ranise C, Sabbioneda S. Maffia A, et al. Int J Mol Sci. 2020 Feb 22;21(4):1506. doi: 10.3390/ijms21041506. Int J Mol Sci. 2020. PMID: 32098397 Free PMC article. Review. - On the wrong DNA track: Molecular mechanisms of repeat-mediated genome instability.
Khristich AN, Mirkin SM. Khristich AN, et al. J Biol Chem. 2020 Mar 27;295(13):4134-4170. doi: 10.1074/jbc.REV119.007678. Epub 2020 Feb 14. J Biol Chem. 2020. PMID: 32060097 Free PMC article. Review. - Expanded GAA repeats impede transcription elongation through the FXN gene and induce transcriptional silencing that is restricted to the FXN locus.
Li Y, Lu Y, Polak U, Lin K, Shen J, Farmer J, Seyer L, Bhalla AD, Rozwadowska N, Lynch DR, Butler JS, Napierala M. Li Y, et al. Hum Mol Genet. 2015 Dec 15;24(24):6932-43. doi: 10.1093/hmg/ddv397. Epub 2015 Sep 23. Hum Mol Genet. 2015. PMID: 26401053 Free PMC article.
References
- Jiang M, Ma N, Vassylyev DG, McAllister WT. RNA displacement and resolution of the transcription bubble during transcription by T7 RNA polymerase. Mol. Cell. 2004;15:777–788. - PubMed
- Datta K, Johnson NP, von Hippel PH. Mapping the conformation of the nucleic acid framework of the T7 RNA polymerase elongation complex in solution using low-energy CD and fluorescence spectroscopy. J. Mol. Biol. 2006;360:800–813. - PubMed
- Naryshkina T, Kuznedelov K, Severinov K. The role of the largest RNA polymerase subunit lid element in preventing the formation of extended RNA-DNA hybrid. J. Mol. Biol. 2006;361:634–643. - PubMed
- Toulokhonov I, Landick R. The role of the lid element in transcription by E. coli RNA polymerase. J. Mol. Biol. 2006;361:644–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous