Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits - PubMed (original) (raw)
. 2007 Aug 21;104(34):13798-803.
doi: 10.1073/pnas.0702553104. Epub 2007 Aug 10.
Thiruma V Arumugam, Xiangru Xu, Aiwu Cheng, Mohamed R Mughal, Dong Gyu Jo, Justin D Lathia, Dominic A Siler, Srinivasulu Chigurupati, Xin Ouyang, Tim Magnus, Simonetta Camandola, Mark P Mattson
Affiliations
- PMID: 17693552
- PMCID: PMC1959462
- DOI: 10.1073/pnas.0702553104
Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits
Sung-Chun Tang et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The innate immune system senses the invasion of pathogenic microorganisms and tissue injury through Toll-like receptors (TLR), a mechanism thought to be limited to immune cells. We now report that neurons express several TLRs, and that the levels of TLR2 and -4 are increased in neurons in response to IFN-gamma stimulation and energy deprivation. Neurons from both TLR2 knockout and -4 mutant mice were protected against energy deprivation-induced cell death, which was associated with decreased activation of a proapoptotic signaling cascade involving jun N-terminal kinase and the transcription factor AP-1. TLR2 and -4 expression was increased in cerebral cortical neurons in response to ischemia/reperfusion injury, and the amount of brain damage and neurological deficits caused by a stroke were significantly less in mice deficient in TLR2 or -4 compared with WT control mice. Our findings establish a proapoptotic signaling pathway for TLR2 and -4 in neurons that may render them vulnerable to ischemic death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
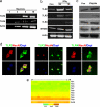
Neurons express TLRs and respond to IFNγ stimulation. (a) TLR2 and -4 mRNA are present in cultured cortical neurons determined by single-cell RT-PCR analysis. The numbers indicate the number of neurons from which RNA was amplified; 3, 6, and 10 cortical neurons consistently yielded a positive PCR signal for the TLR2 and -4 with exactly predicted size. (b) Level of TLR mRNAs in neurons treated with IFNγ (500 μg/ml). Neurons expressed TLR2, -3, -4, and MYD88 mRNAs, and their expression levels increased in response to IFNγ. (c) Immunoblot showing relative levels of TLR2 (95 kDa), TLR3 (95 kDa), TLR4 (90 kDa), and MYD88 (33 kDa) in lysates of cultured neurons treated with vehicle (Con) or IFNγ. (d) TLR2, -3, and -4 immunoreactivities (green) in cultured neurons; cells were counterstained with DAPI (blue) to label all nuclei and with the neuron-specific marker NeuN (red). (e) Results of microarray analysis for the indicated TLR mRNAs in RNA samples isolated from cultured cortical neurons (six separate RNA samples run in triplicate; c1–c18). Relative expression levels are indicated by the color scale shown.
Fig. 2.
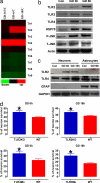
Expression of TLR2 and -4 increases after GD, and neurons deficient in TLR2 or -4 receptors are resistant to GD-induced death. (a) Results of a microarray analysis comparing levels of the indicated TLR mRNAs in neurons in control cultures with the level in cultures subjected to GD for 0.5, 6, or 18 h. Note that GD induced increased expression of TLR2 and -4 but not the other TLRs. (b) Immunoblot analysis of proteins in cell lysates of neurons in control cultures and cultures subjected to GD for 3 or 6 h. GD resulted in increased levels of TLR2, -4, HSP70, and _p_-JNK. (c) Immunoblot analysis of proteins in cell lysates of neurons or astrocytes in control cultures and cultures subjected to GD for the indicated time periods. (d) Cultured cortical neurons from WT mice, TLR2 knockout mice, and TLR4 mutant mice were subjected to GD for the indicated time periods, and cell survival was quantified. Values are the mean and SEM (n = 24–36 cultures). *, P < 0.05 compared with the WT value.
Fig. 3.
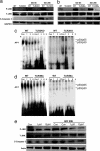
TLR2 and -4 mediate energy deprivation-induced activation of the JNK-AP-1 pathway and caspase-3 in cultured cortical neurons. (a and b) Immunoblot analysis of proteins in cell lysates of neurons from WT, TLR2 knockout, and TLR4 mutant mice that had been subjected to GD for the indicated time periods. GD resulted in increased levels of _p_-JNK and the cleaved form of caspase-3 (19-kDa) in WT neurons but little or no increase in _p_-JNK or activated caspase-3 in neurons deficient in TLR2 or -4. (c and d) Gel-shift analysis showing NF-κB and AP-1 DNA-binding activities in lysates of neurons from WT, TLR2 knockout mice, or TLR4 mutant mice that had been subjected to GD for 1, 3, or 6 h. Levels of AP-1 DNA-binding activity were lower in samples from TLR2 and -4 mutant mice compared with WT mice. (e) Neuronal cultures were treated with 1 or 10 μM of the JNK inhibitor SP600125 and then subjected to GD (or not) for 20 h. Proteins in cell lysates were then subjected to immunoblot analysis by using the indicated antibodies. The JNK inhibitor attenuated GD-induced increases in levels of _p_-JNK and activated caspase-3.
Fig. 4.
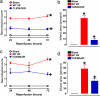
TLR2 and -4 mediate ischemic neuronal death and functional deficits in a mouse stroke model. Mice of the indicated genotypes were subjected to sham surgery or ischemia/reperfusion (I/R), neurological function was evaluated daily for 3 d, and brain damage was evaluated at 3 d. (a and b) Neurological scores (a) and infarct volumes (b) for WT mice subjected to sham surgery (n = 5), and WT (n = 12) and TLR2 knockout (n = 11) mice subjected to I/R. *, P < 0.001 compared with the sham value. +, P < 0.01 compared with the WT I/R value. (c and d) Neurological scores (c) and infarct volumes (d) for WT mice subjected to sham surgery (n = 5), and WT (n = 8) and TLR4 mutant (n = 7) mice subjected to I/R. *, P < 0.001 compared with the sham value. +, P < 0.01 compared with the WT I/R value.
Fig. 5.
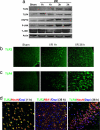
Cerebral ischemia induces a rapid increase in TLR2 and -4 immunoreactivities in neurons and a delayed appearance of TLR2-positive microglia. (a) Immunoblot analysis of protein samples from the cerebral cortex of sham-operated control mice and mice killed either 1 or 3 h after I/R. (b–d) Images of brain sections showing TLR2 and -4 immunoreactivities (green), DAPI staining (blue), and NeuN (neuronal marker) or IBA1 (microglial marker) immunoreactivities (red) in WT mice subjected to sham surgery of I/R for the indicated time periods. Ischemia resulted in rapid increases in the levels of TLR2 and -4 immunoreactivities in neurons in the cerebral cortex and a delayed appearance of TLR2 immunoreactive IBA1-positive microglial cells.
Similar articles
- Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice.
Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, Kalbfleisch JH, Williams DL, Li C. Hua F, et al. Brain Res. 2009 Mar 25;1262:100-8. doi: 10.1016/j.brainres.2009.01.018. Epub 2009 Jan 22. Brain Res. 2009. PMID: 19401158 Free PMC article. - Role of baicalin in regulating Toll-like receptor 2/4 after ischemic neuronal injury.
Li HY, Yuan ZY, Wang YG, Wan HJ, Hu J, Chai YS, Lei F, Xing DM, DU LJ. Li HY, et al. Chin Med J (Engl). 2012 May;125(9):1586-93. Chin Med J (Engl). 2012. PMID: 22800826 - Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal.
Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Tang SC, et al. Exp Neurol. 2008 Sep;213(1):114-21. doi: 10.1016/j.expneurol.2008.05.014. Epub 2008 May 29. Exp Neurol. 2008. PMID: 18586243 Free PMC article. - TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion.
Wang Y, Ge P, Zhu Y. Wang Y, et al. Mediators Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. Epub 2013 Jun 23. Mediators Inflamm. 2013. PMID: 23864765 Free PMC article. Review. - Involvement of Toll-like receptors in ischemia-induced neuronal damage.
Hamanaka J, Hara H. Hamanaka J, et al. Cent Nerv Syst Agents Med Chem. 2011 Jun 1;11(2):107-13. doi: 10.2174/187152411796011312. Cent Nerv Syst Agents Med Chem. 2011. PMID: 21521172 Review.
Cited by
- Evidence for a developmental role for TLR4 in learning and memory.
Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Castro K, Mughal MR, Pita MA, Stranahan AM, Arumugam TV, Mattson MP. Okun E, et al. PLoS One. 2012;7(10):e47522. doi: 10.1371/journal.pone.0047522. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071817 Free PMC article. - Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia.
Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, Ishiguro M, Tsuruma K, Hirose Y, Tanaka H, Yoshimura S, Shimazawa M, Inagaki N, Nagasawa H, Iwama T, Hara H. Suzuki Y, et al. Sci Rep. 2012;2:896. doi: 10.1038/srep00896. Epub 2012 Nov 28. Sci Rep. 2012. PMID: 23193438 Free PMC article. - Screening of Toll-like receptors expression in multiple system atrophy brains.
Brudek T, Winge K, Agander TK, Pakkenberg B. Brudek T, et al. Neurochem Res. 2013 Jun;38(6):1252-9. doi: 10.1007/s11064-013-1020-5. Epub 2013 Mar 23. Neurochem Res. 2013. PMID: 23525968 - Increased expression of toll-like receptor 4 on neurons after surgery in aged rats.
Wang Y, He HJ, Ouyang W. Wang Y, et al. CNS Neurosci Ther. 2013 May;19(5):358-60. doi: 10.1111/cns.12090. Epub 2013 Mar 28. CNS Neurosci Ther. 2013. PMID: 23534838 Free PMC article. No abstract available. - Neuroimmune signaling: a key component of alcohol abuse.
Mayfield J, Ferguson L, Harris RA. Mayfield J, et al. Curr Opin Neurobiol. 2013 Aug;23(4):513-20. doi: 10.1016/j.conb.2013.01.024. Epub 2013 Feb 22. Curr Opin Neurobiol. 2013. PMID: 23434064 Free PMC article. Review.
References
- Akira S. Curr Top Microbiol Immunol. 2006;311:1–16. - PubMed
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al. Nature. 2001;413:78–83. - PubMed
- Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. FEBS Lett. 2003;548:103–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous