The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? - PubMed (original) (raw)
Review
The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells?
Steven P Arnoczky et al. Int J Exp Pathol. 2007 Aug.
Abstract
While there is a significant amount of information available on the clinical presentation(s) and pathological changes associated with tendinopathy, the precise aetiopathogenesis of this condition remains a topic of debate. Classically, the aetiology of tendinopathy has been linked to the performance of repetitive activities (so-called overuse injuries). This has led many investigators to suggest that it is the mechanobiologic over-stimulation of tendon cells that is the initial stimulus for the degradative processes which have been shown to accompany tendinopathy. Although several studies have been able to demonstrate that the in vitro over-stimulation of tendon cells in monolayer can result in a pattern(s) of gene expression seen in clinical cases of tendinopathy, the strain magnitudes and durations used in these in vitro studies, as well as the model systems, may not be clinically relevant. Using a rat tail tendon model, we have studied the in vitro mechanobiologic response of tendon cells in situ to various tensile loading regimes. These studies have led to the hypothesis that the aetiopathogenic stimulus for the degenerative cascade which precedes the overt pathologic development of tendinopathy is the catabolic response of tendon cells to mechanobiologic under-stimulation as a result of microscopic damage to the collagen fibres of the tendon. In this review, we examine the rationale for this hypothesis and provide evidence in support of this theory.
Figures
Figure 1
Proposed algorithm of the aetiopathogenesis of tendinopathy. Modified from Archambault et al. 1995; Arnoczky et al. 2007.
Figure 2
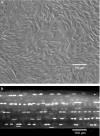
(a) Phase contrast, microscopic image of rat tail tendon cells in monolayer. The random orientation, density, and number of the cells does not replicate the normal in situ cellular environment. (b) Confocal laser microscopic image of a rat tail tendon fascicle stained with acridine orange. Using this model system, the natural distribution, number, and orientation of the tendon cells within their normal extracellular matrix is maintained.
Figure 3
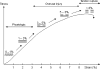
Schematic drawing of a load deformation curve illustrating the mechanical response of a tendon to tensile loading. Modified from Józsa & Kannus 1997; Arnoczky et al. 2007.
Figure 4
Time-lapse confocal images of a rat tail tendon being strained at a rate of 20 μm/s. The cell nuclei have been stained with acridine orange and the pairs of short and long arrows identify cell nuclei used as fiduciary markers to demonstrate fibril sliding. At 7% strain (image 3) the lower (long) pair of arrows can be seen separating indicating fibre slippage. This separation continues to increase with increasing strain (images 4 and 5). After 8% strain (image 4) the upper (short) pair of arrows begin to get closer and pass over one another at 9% strain (image 5). This slippage continues to increase at 10% strain (image 6). In both instances, fibril slippage occurred in advance of complete tendon rupture (Arnoczky et al. 2007).
Figure 5
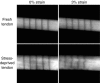
Confocal images of a fresh and 21-day stress deprived rat tail tendon. Parallel registration lines have been photobleached onto the surface of the tendons. When strained to 3% (grip-to-grip strain) the registration lines on the fresh tendon remain parallel. This is in contrast to the 21-day stress deprived tendon that demonstrated an altered strain pattern due to breakdown of the extracellular matrix by collagenase which is upregulated in these tendons following stress-deprivation (Arnoczky et al. 2007).
Figure 6
Images of a rat tail tendon fascicle at various points throughout the testing protocol: (a) prior to loading (the crimp pattern is clearly visible), (b) during loading in the linear portion of the stress–strain curve demonstrating the elimination of the crimp pattern, (c) onset of fibrillar damage as manifested by a change in the reflectivity of the damaged fibrils (arrows), and (d) unloading of the tendon to 100 g and the reoccurrence of the crimp pattern within the damaged fibrils (arrows). (bar = 200 μm). (Reprinted from Lavagnino et al. (2006a) Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J. Biomech. 39, 2355–2362, with permission from Elsevier.)
Figure 7
Representative images of a rat tail tendon fascicle following fibrillar damage. (a) Presence of the crimp pattern on the bottom of the tendon fascicle (arrows) indicates the site of isolated fibrillar damage. (b) In situ hybridization of the tendon fascicle reveals interstitial collagenase mRNA expression in those cells associated with the damaged fibril(s). The borders of the tendon fascicle are delineated by lines. (bar = 100 μm). Reprinted from Lavagnino et al. (2006a). Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J. Biomech. 39, 2355–2362, with permission from Elsevier.)
Figure 8
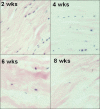
Photomicrographs of the histological changes seen in canine flexor digitorum profundus tendons following stress-deprivation for 2, 4, 6 and 8 weeks. Note how the tendon cells change morphology and ‘round up’ in response to the loss of normal homeostatic tension. Also note the progressive decrease in cell number and the progressive disruption of the collagen architecture with time of stress-deprivation. (H & E ×100)
Similar articles
- Strain patterns in the patellar tendon and the implications for patellar tendinopathy.
Almekinders LC, Vellema JH, Weinhold PS. Almekinders LC, et al. Knee Surg Sports Traumatol Arthrosc. 2002 Jan;10(1):2-5. doi: 10.1007/s001670100224. Epub 2001 Aug 16. Knee Surg Sports Traumatol Arthrosc. 2002. PMID: 11819013 - Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells.
Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM. Rui YF, et al. J Orthop Res. 2011 Mar;29(3):390-6. doi: 10.1002/jor.21218. Epub 2010 Sep 29. J Orthop Res. 2011. PMID: 20882582 - Is compressive load a factor in the development of tendinopathy?
Cook JL, Purdam C. Cook JL, et al. Br J Sports Med. 2012 Mar;46(3):163-8. doi: 10.1136/bjsports-2011-090414. Epub 2011 Nov 22. Br J Sports Med. 2012. PMID: 22113234 Review. - Overuse tendonitis and rehabilitation.
Giffin JR, Stanish WD. Giffin JR, et al. Can Fam Physician. 1993 Aug;39:1762-9. Can Fam Physician. 1993. PMID: 8374363 Free PMC article. Review.
Cited by
- Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ.
Jones ER, Jones GC, Legerlotz K, Riley GP. Jones ER, et al. Biochim Biophys Acta. 2013 Dec;1833(12):2596-2607. doi: 10.1016/j.bbamcr.2013.06.019. Epub 2013 Jul 2. Biochim Biophys Acta. 2013. PMID: 23830915 Free PMC article. - Models of tendon development and injury.
Theodossiou SK, Schiele NR. Theodossiou SK, et al. BMC Biomed Eng. 2019;1:32. doi: 10.1186/s42490-019-0029-5. Epub 2019 Nov 29. BMC Biomed Eng. 2019. PMID: 32095779 Free PMC article. - Structure and collagen crimp patterns of functionally distinct equine tendons, revealed by quantitative polarised light microscopy (qPLM).
Spiesz EM, Thorpe CT, Thurner PJ, Screen HRC. Spiesz EM, et al. Acta Biomater. 2018 Apr 1;70:281-292. doi: 10.1016/j.actbio.2018.01.034. Epub 2018 Feb 2. Acta Biomater. 2018. PMID: 29409868 Free PMC article. - The role of mechanical loading in tendon development, maintenance, injury, and repair.
Galloway MT, Lalley AL, Shearn JT. Galloway MT, et al. J Bone Joint Surg Am. 2013 Sep 4;95(17):1620-8. doi: 10.2106/JBJS.L.01004. J Bone Joint Surg Am. 2013. PMID: 24005204 Free PMC article. Review. - In Vitro Innovation of Tendon Tissue Engineering Strategies.
Citeroni MR, Ciardulli MC, Russo V, Della Porta G, Mauro A, El Khatib M, Di Mattia M, Galesso D, Barbera C, Forsyth NR, Maffulli N, Barboni B. Citeroni MR, et al. Int J Mol Sci. 2020 Sep 14;21(18):6726. doi: 10.3390/ijms21186726. Int J Mol Sci. 2020. PMID: 32937830 Free PMC article. Review.
References
- Alfredson H, Lorentzon M, Bäckman S, Bäckman A, Lerner UH. cDNA arrays and real time qualitative PCR techniques in the investigation of chronic Achilles tendinosis. J. Orthop. Res. 2003;21:970–975. - PubMed
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. - PubMed
- Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. Sports Med. 1995;20:77–89. - PubMed
- Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect. Tissue Res. 2001;42:13–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical