Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium - PubMed (original) (raw)
. 2007 Aug 13;178(4):635-48.
doi: 10.1083/jcb.200704152.
Charbel Darido, Julie Pannequin, Ralf Kist, Sylvie Robine, Christiane Marty-Double, Frédéric Bibeau, Gerd Scherer, Dominique Joubert, Frédéric Hollande, Philippe Blache, Philippe Jay
Affiliations
- PMID: 17698607
- PMCID: PMC2064470
- DOI: 10.1083/jcb.200704152
Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium
Pauline Bastide et al. J Cell Biol. 2007.
Abstract
The HMG-box transcription factor Sox9 is expressed in the intestinal epithelium, specifically, in stem/progenitor cells and in Paneth cells. Sox9 expression requires an active beta-catenin-Tcf complex, the transcriptional effector of the Wnt pathway. This pathway is critical for numerous aspects of the intestinal epithelium physiopathology, but processes that specify the cell response to such multipotential signals still remain to be identified. We inactivated the Sox9 gene in the intestinal epithelium to analyze its physiological function. Sox9 inactivation affected differentiation throughout the intestinal epithelium, with a disappearance of Paneth cells and a decrease of the goblet cell lineage. Additionally, the morphology of the colon epithelium was severely altered. We detected general hyperplasia and local crypt dysplasia in the intestine, and Wnt pathway target genes were up-regulated. These results highlight the central position of Sox9 as both a transcriptional target and a regulator of the Wnt pathway in the regulation of intestinal epithelium homeostasis.
Figures
Figure 1.
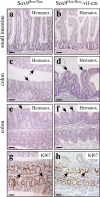
Absence of Sox9 protein expression in the intestinal epithelium of Sox9-deficient mice. In Sox9flox/flox mice, the Sox9 protein is expressed in the bottom of small intestinal (a) and colon (c) crypts. The absence of specific Sox9 staining in the small intestine (b) and colon (d) from Sox9flox/flox-vil-Cre mice demonstrate efficient vil-Cre–mediated recombination. Arrows and arrowheads indicate Sox9-positive and -negative nuclei, respectively. Bars: (panels) 150 μm; (insets) 50 μm.
Figure 2.
Morphological alterations in the colon of Sox9-deficient mice. Histological analysis (hematoxylin staining) of the intestine of Sox9flox/flox (a, c, and e) and Sox9flox/flox-vil-Cre mice (b, d, and f). The gross morphology of the small intestine of the Sox9flox/flox-vil-Cre mice is not affected (a and b), whereas that of the colon is strongly altered. The surface of the colon is normally flat (c and e, arrows), but villus-like structures protrude into the lumen of the colon of Sox9flox/flox-vil-Cre mice (d and f, arrows). Immunohistochemical staining with the Ki-67 proliferation in Sox9flox/flox mice (g) and Sox9flox/flox-vil-Cre mice (h) indicates appropriate crypt-restricted proliferation in the colon of Sox9-deficient animals. Bars, 150 μm.
Figure 3.
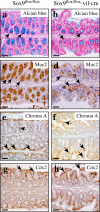
Altered goblet cell differentiation in the colon of Sox9-deficient mice. Immunohistochemical analysis of Sox9flox/flox mice (a, c, e, and g) and Sox9flox/flox-vil-Cre mice (b, d, f, and h). Alcian blue staining (a and b) and Muc2 immunoreactivity (c and d) reveal a strong reduction of the goblet cell population in the colon epithelium of Sox9flox/flox-vil-Cre mice. Sox9flox/flox and Sox9flox/flox-vil-Cre mice have comparable populations of chromogranin A–positive enteroendocrine cells (e and f) and Cdx-2–positive enterocytes (g and h). Bars, 150 μm.
Figure 4.
Sox9 controls differentiation of the Paneth and goblet cell lineages. Sox9flox/flox (a, c, e, g, i, k, and m) and Sox9flox/flox-vil-Cre (b, d, f, h, j, l, and n) mice are compared. Differentiation along the enterocyte (a and b) and enteroendocrine (c and d) lineages is not affected. The goblet cell population, revealed by alcian blue staining for acidic mucins (e and f) and Muc2 immunostaining (g and h), is reduced in the small intestinal epithelium of Sox9-deficient mice. Paneth cells (arrows in control mice) are completely absent (arrows) in Sox9-deficient crypts (i and j). Instead, the proliferative compartment (k–n, arrows) expands to occupy the whole crypt bottom in mutant animals, where more Musashi-1–expressing putative stem cells are found (o and p, arrows). Ectopic Paneth cell found in a human patient with Barrett's esophagus (intestinal metaplasia in the esophagus) also express Sox9, as shown by staining of adjacent sections with lysozyme (q) and Sox9 (r) antibodies. Expression analysis of the Paneth cell markers lysozyme, MMP7, and Angiogenin-4, in the HT29Cl.16E-Sox9 cell line, before and after doxycycline induction of exogenous Sox9 expression (s). Bars: (a–l, q, and r) 120 μm; (m and n) 30 μm; (o and p) 40 μm.
Figure 5.
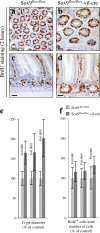
Generalized hyperplasia develops in the absence of Sox9. Difference of crypt size in the small intestines of Sox9flox/flox (a and c) and Sox9flox/flox-vil-Cre (b and d) mice. (a and b) Crypt cross sections; (c and d) crypt longitudinal sections. Bars, 75 μm. (e) Histogram showing mean crypt diameters along the small intestines of three Sox9flox/flox and three Sox9flox/flox-vil-Cre mice. Standard deviations are indicated. P < 0.001 (t test). (f) Histogram showing the BrdU incorporation rates (number of BrdU-positive cells/total number of cells) in crypts from three Sox9flox/flox and three Sox9flox/flox-vil-Cre mice. Standard deviations are indicated. P < 0.001 (t test).
Figure 6.
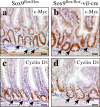
Sox9 deficiency causes hyperplasia and dysplasia. In the colon epithelium of Sox9flox/flox-vil-Cre mice, hyperplastic lesions are found (hematoxylin staining) with branched and enlarged crypts (a). Some crypts have a cystic appearance (b). Such structures proliferate (c and d, arrows). Tubulovillous microadenomas spontaneously developed in hyperplastic areas of Sox9flox/flox-vil-Cre mice (e). Typical example of the dysplastic-looking crypts (arrows) found in the distal half of the colon of Sox9-deficient mice. Arrowheads point at mutiadenoid crypts (f). Compared with normal tissue (g, i, and k), hyperplastic and dysplastic crypts of Sox9-deficient mice (h, j, and l) overexpress Wnt pathway target genes such as c-Myc (compare g and h) and cyclin D1 (compare i and j) and, accordingly, have elevated staining for the Ki67 proliferation marker (compare k and l). Insets in panels g–l show enlarged pictures of the indicated area of the panel, and arrows point at typical staining pictures. Western blot analysis of c-Myc, cyclin D1, and PCNA in the small intestine and colon of Sox9flox/flox and Sox9flox/flox-vil-Cre mice. β-Actin expression is shown as a loading control (m). Stool hydration is increased in Sox9-deficient mice (n). Bars: (panels) 150 μm; (insets) 50 μm.
Figure 7.
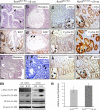
Sox9 fine-tunes the activity of the β-catenin–Tcf4 transcriptional effector of the Wnt signaling pathway. (a and b) No alteration of the number of cells containing nuclear β-catenin (arrows) is found in Sox9-deficient animals. Bars, 40 μm. (c and d) Analysis of the transcriptional activity of the β-catenin–Tcf4 complex, using a luciferase reporter system (Morin et al., 1997). Endogenous transcriptional activity of the β-catenin–Tcf4 complex. Transient overexpression of Sox9 inhibits the β-catenin–Tcf4 activity, whereas overexpressing ΔCSox9 increases it (c). Similarily, induction of Sox9 overexpression in the HT29-16E-Sox9 cell line causes inhibition of β-catenin–Tcf4 transcriptional activity, whereas inducing overexpression of ΔCSox9 in the HT29-16E-ΔCSox9 cell line resulted in a considerable increase of this activity (d). Regulation of the β-catenin–Tcf4 transcriptional activity impacted c-Myc and cyclin D1 mRNA expression. The amounts of mRNA after doxycycline induction are indicated as a percentage of the noninduced state (e, dashed line). These variations at the mRNA level were reflected at the protein level (f).
Figure 8.
Sox9 transcriptionally activates expression of inhibitors of the β-catenin–Tcf activity. Structure–function analysis of the inhibitory function of Sox9 on the β-catenin–Tcf activity (a–e) in DLD-1 cells. (a) Diagram showing the different constructs used in this study. (b) Transcriptional activity of Sox9 and its mutated or truncated versions on a Sox-luciferase reporter system. (c) Inhibition of the β-catenin–Tcf activity by Sox9 and its truncated or mutated versions. (d) Transcriptional activity of the Sox9-VP16 chimeric protein compared with that of Sox9 on a Sox-luciferase reporter system. (e) Inhibition of the β-catenin–Tcf activity by Sox9 and Sox9-VP16. In panels b and d, the activity of the wild-type Sox9 construct is arbitrarily set to 100. In panels c and e, the endogenous β-catenin–Tcf activity is arbitrarily set to 100. (f and g) Real-time RT-PCR analysis of the expression of the inhibitor of β-catenin and Tcf (ICAT) and Groucho-related inhibitors of the β-catenin–Tcf activity in HT29Cl.16E-Sox9 cells before and after induction of exogenous Sox9 expression (f) and in the intestinal mucosa of Sox9flox/flox versus Sox9flox/flox-vil-Cre mice (g). Results are expressed relative to the noninduced or nonrecombined states. Standard deviations are indicated.
Figure 9.
Alteration of c-Myc and cyclin D1 expression in the bottom of Sox9-deficient crypts. C-Myc and cyclin D1 expression was analyzed by immunohistochemistry in the intestine of Sox9flox/flox (a and c) and Sox9flox/flox- vil-Cre (b and d) mice. Arrows indicate the Paneth cell compartment in Sox9flox/flox control mice (a and c) and the equivalent location in the Sox9flox/flox-vil-Cre mice (b and d). Bars, 50 μm.
Similar articles
- SOX9 is required for the differentiation of paneth cells in the intestinal epithelium.
Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. Mori-Akiyama Y, et al. Gastroenterology. 2007 Aug;133(2):539-46. doi: 10.1053/j.gastro.2007.05.020. Epub 2007 May 21. Gastroenterology. 2007. PMID: 17681175 - SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes.
Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. Blache P, et al. J Cell Biol. 2004 Jul 5;166(1):37-47. doi: 10.1083/jcb.200311021. J Cell Biol. 2004. PMID: 15240568 Free PMC article. - Sox9 induction, ectopic Paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation.
Feng Y, Sentani K, Wiese A, Sands E, Green M, Bommer GT, Cho KR, Fearon ER. Feng Y, et al. Am J Pathol. 2013 Aug;183(2):493-503. doi: 10.1016/j.ajpath.2013.04.013. Epub 2013 Jun 13. Am J Pathol. 2013. PMID: 23769888 Free PMC article. - The role of SOX9 transcription factor in pancreatic and duodenal development.
Belo J, Krishnamurthy M, Oakie A, Wang R. Belo J, et al. Stem Cells Dev. 2013 Nov 15;22(22):2935-43. doi: 10.1089/scd.2013.0106. Epub 2013 Aug 2. Stem Cells Dev. 2013. PMID: 23806070 Review. - The Yin-Yang of TCF/beta-catenin signaling.
Barker N, Morin PJ, Clevers H. Barker N, et al. Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
Cited by
- Metastasis of colon cancer requires Dickkopf-2 to generate cancer cells with Paneth cell properties.
Shin JH, Park J, Lim J, Jeong J, Dinesh RK, Maher SE, Kim J, Park S, Hong JY, Wysolmerski J, Choi J, Bothwell ALM. Shin JH, et al. Elife. 2024 Nov 13;13:RP97279. doi: 10.7554/eLife.97279. Elife. 2024. PMID: 39535280 Free PMC article. - Transcriptomic Insights into Hub Genes, Immune Infiltration, and Candidate Drugs in Erosive Esophagitis.
Zhao Y, Chen X, Huang Y, Zhang Z, Wang K, Zou D, Ma T. Zhao Y, et al. J Inflamm Res. 2024 Oct 28;17:7745-7760. doi: 10.2147/JIR.S479032. eCollection 2024. J Inflamm Res. 2024. PMID: 39494202 Free PMC article. - Development of an intestinal epithelial cell line and organoids derived from the same swine and characterization of their antiviral responses.
Matsumoto K, Namai F, Miyazaki A, Imamura Y, Fukuyama K, Ikeda-Ohtsubo W, Nishiyama K, Villena J, Miyazawa K, Kitazawa H. Matsumoto K, et al. Biosci Microbiota Food Health. 2024;43(4):342-351. doi: 10.12938/bmfh.2024-0046. Epub 2024 May 28. Biosci Microbiota Food Health. 2024. PMID: 39364127 Free PMC article. - Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview.
Dias Da Silva I, Wuidar V, Zielonka M, Pequeux C. Dias Da Silva I, et al. Cells. 2024 Jul 23;13(15):1236. doi: 10.3390/cells13151236. Cells. 2024. PMID: 39120268 Free PMC article. Review. - Interaction between microRNA-195 and HuR regulates Paneth cell function in the intestinal epithelium by altering SOX9 translation.
Kwon MS, Chung HK, Xiao L, Yu TX, Sharma S, Cairns CM, Chen T, Chae S, Turner DJ, Wang JY. Kwon MS, et al. Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C817-C829. doi: 10.1152/ajpcell.00325.2024. Epub 2024 Aug 5. Am J Physiol Cell Physiol. 2024. PMID: 39099425
References
- Afonja, O., B.M. Raaka, A. Huang, S. Das, X. Zhao, E. Helmer, D. Juste, and H.H. Samuels. 2002. RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: evidence for a role in retinoid-mediated growth inhibition. Oncogene. 21:7850–7860. - PubMed
- Andreu, P., S. Colnot, C. Godard, S. Gad, P. Chafey, M. Niwa-Kawakita, P. Laurent-Puig, A. Kahn, S. Robine, C. Perret, and B. Romagnolo. 2005. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 132:1443–1451. - PubMed
- Batlle, E., J.T. Henderson, H. Beghtel, M.M.W. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell. 111:251–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous