Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate - PubMed (original) (raw)
Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate
Jem A Efe et al. Mol Biol Cell. 2007 Nov.
Abstract
The lipid kinase Fab1 governs yeast vacuole homeostasis by generating PtdIns(3,5)P(2) on the vacuolar membrane. Recruitment of effector proteins by the phospholipid ensures precise regulation of vacuole morphology and function. Cells lacking the effector Atg18p have enlarged vacuoles and high PtdIns(3,5)P(2) levels. Although Atg18 colocalizes with Fab1p, it likely does not directly interact with Fab1p, as deletion of either kinase activator-VAC7 or VAC14-is epistatic to atg18Delta: atg18Deltavac7Delta cells have no detectable PtdIns(3,5)P(2). Moreover, a 2xAtg18 (tandem fusion) construct localizes to the vacuole membrane in the absence of PtdIns(3,5)P(2), but requires Vac7p for recruitment. Like the endosomal PtdIns(3)P effector EEA1, Atg18 membrane binding may require a protein component. When the lipid requirement is bypassed by fusing Atg18 to ALP, a vacuolar transmembrane protein, vac14Delta vacuoles regain normal morphology. Rescue is independent of PtdIns(3,5)P(2), as mutation of the phospholipid-binding site in Atg18 does not prevent vacuole fission and properly regulates Fab1p activity. Finally, the vacuole-specific type-V myosin adapter Vac17p interacts with Atg18p, perhaps mediating cytoskeletal attachment during retrograde transport. Atg18p is likely a PtdIns(3,5)P(2)"sensor," acting as an effector to remodel membranes as well as regulating its synthesis via feedback that might involve Vac7p.
Figures
Figure 1.
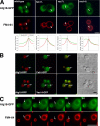
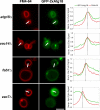
Atg18 is entirely cytosolic in _fab1_Δ, _vac7_Δ, and _vac14_Δ mutants. (A) Fluorescence microscopy was used to determine the localization of an Atg18-GFP fusion in relation to the vacuoles of wild-type and mutant cells, as labeled with FM4-64. Overlap with the vacuolar membrane was quantified with ImageJ software by plotting normalized fluorescence intensity along a path traversing the vacuole membrane (indicated by the white bars). For orientation purposes, the cytosol (left) is separated from the lumen of the vacuole (right) by a dotted line. (B) Atg18-RFP localizes to puncta on the vacuolar rim that partially overlap with Vac14-GFP and Fab1-GFP. RFP/GFP pairs were coexpressed and their localization was compared by fluorescence microscopy. Arrows in the merge panels indicate areas of extensive overlap. (C) Atg18-GFP puncta are highly mobile. Representative still images from a 64-s time-lapse movie (Supplementary Movie 1; figure 1C.mov) depict a patch of membrane enriched in Atg18-GFP (arrows) budding from the mother cell vacuole and traveling into the daughter cell. Simultaneously acquired FM4-64 fluorescence images highlight the vacuolar membranes. Bars, 4 μm.
Figure 2.
Deletion of FIG 4, VAC7, or VAC14 is epistatic to that of ATG18. PtdIns(3)P and PtdIns(3,5)_P_2 levels in (A) _vac7_Δ/_atg18_Δ and _vac14_Δ/_atg18_Δ or (B) _atg18_Δ/_fig4_Δ strains were analyzed and compared with those of the single mutant parents and a wild-type strain. 3H-labeled phosphoinositides were isolated and measured by HPLC as described in Materials and Methods.
Figure 3.
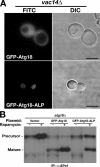
A GFP-Atg18-ALP fusion restores wild-type vacuole morphology in a _vac14_Δ strain. (A) Fluorescence microscopy comparison of GFP-Atg18 and GFP-Atg18-ALP fusion protein localization and the resulting vacuole morphology in a _vac14_Δ/_atg18_Δ strain. Bars, 4 μm. (B) GFP-Atg18-ALP is defective in the cytoplasm-to-vacuole and macroautophagy pathways. APe1 was immunoprecipitated from whole cell lysates of cells metabolically labeled with [35S]methionine (chase time is 2 h for all samples) in the presence or absence of rapamycin. Proteolytic maturation of APe1 was analyzed by SDS-PAGE.
Figure 4.
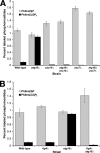
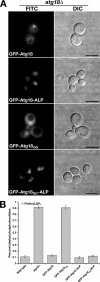
GFP-Atg18-ALP can alleviate _atg18_Δ phenotypes even if the putative PtdIns(3,5)_P_2 binding site is mutated. (A) GFP-Atg18 and GFP-Atg18-ALP fusions harboring either wild-type or an 285RR286-to-285GG286 point mutant of Atg18 were transformed into _atg18_Δ cells and visualized by fluorescence microscopy. Bars, 4 μm. (B) PtdIns(3,5)_P_2 levels in the same set of transformants were measured as described in the legend to Figure 2.
Figure 5.
GFP-2xAtg18 can bind to the vacuole membrane in the absence of PtdIns(3,5)_P_2, but requires Vac7p for membrane localization. _atg18_Δ, _vac14_Δ, _fab1_Δ, and _vac7_Δ cells expressing GFP-2xAtg18 were labeled with the fluorescent dye FM4-64 to highlight vacuoles. Coincidence of the GFP signal with the vacuole membrane was quantified as described in the legend to Figure 1. Bars, 4 μm.
Figure 6.
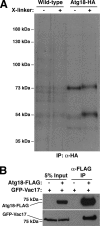
Atg18p interacts with Vac17p in vivo. (A) Osmotic lysates from cells metabolically labeled with [35S]methionine for 1 h were cross-linked with DSP for 30 min and Atg18-HA was immunoprecipitated as described in Materials and Methods. After cleavage of the cross-linker, associated proteins were visualized by SDS-PAGE and subsequent autoradiography. (B) Twenty OD600 units of the indicated strains were spheroplasted and osmotically lysed. Atg18-FLAG was immunoprecipitated from detergent-treated (0.2% Tween-20) whole cell lysates, and association with GFP-Vac17 was determined by SDS-PAGE.
Figure 7.
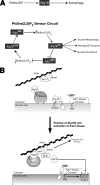
(A) PtdIns(3)P and PtdIns(3,5)_P_2 most likely recruit Atg18 to two distinct membrane compartments, where it is required for autophagy (PAS) and regulation of organelle morphology (vacuole), respectively. On the vacuole, we envision Atg18 acting as a “sensor” of PtdIns(3,5)_P_2, continually cycling between the cytosol (Atg18CYTO) and the limiting membrane (Atg18VAC) in response to changes in phosphoinositide levels. One function of Atg18 on the membrane is to inhibit the Fab1 kinase, thus establishing a negative feedback loop. This circuit forms the integral part of an elegant system that allows dynamic control of vacuole morphology. (B) Model for Atg18 function as it cycles on and off the vacuole membrane. During and after recruitment by the phospholipid PtdIns(3,5)_P_2, Atg18 also associates with Vac7 and Vac17. Atg18 may indirectly regulate activity of the Fab1 kinase by sequestering Vac7. Vac17 most likely functions as a myosin-specific adapter for Atg18, thus enabling retrograde membrane transport from the vacuole along actin tracks. Membrane deformation could be directly or indirectly mediated by Atg18, e.g., via recruitment of an as-of-yet unidentified fission factor.
Similar articles
- Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase.
Rudge SA, Anderson DM, Emr SD. Rudge SA, et al. Mol Biol Cell. 2004 Jan;15(1):24-36. doi: 10.1091/mbc.e03-05-0297. Epub 2003 Oct 3. Mol Biol Cell. 2004. PMID: 14528018 Free PMC article. - Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member.
Gary JD, Sato TK, Stefan CJ, Bonangelino CJ, Weisman LS, Emr SD. Gary JD, et al. Mol Biol Cell. 2002 Apr;13(4):1238-51. doi: 10.1091/mbc.01-10-0498. Mol Biol Cell. 2002. PMID: 11950935 Free PMC article. - Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis.
Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Gary JD, et al. J Cell Biol. 1998 Oct 5;143(1):65-79. doi: 10.1083/jcb.143.1.65. J Cell Biol. 1998. PMID: 9763421 Free PMC article. - The Fab1 phosphatidylinositol kinase pathway in the regulation of vacuole morphology.
Efe JA, Botelho RJ, Emr SD. Efe JA, et al. Curr Opin Cell Biol. 2005 Aug;17(4):402-8. doi: 10.1016/j.ceb.2005.06.002. Curr Opin Cell Biol. 2005. PMID: 15975782 Review. - Phosphoinositide signaling: vac to the future in fab1 kinase regulation.
DeWald DB. DeWald DB. Curr Biol. 2002 Jul 23;12(14):R491-2. doi: 10.1016/s0960-9822(02)00966-1. Curr Biol. 2002. PMID: 12176349 Review.
Cited by
- Structural and functional characterization of the two phosphoinositide binding sites of PROPPINs, a β-propeller protein family.
Krick R, Busse RA, Scacioc A, Stephan M, Janshoff A, Thumm M, Kühnel K. Krick R, et al. Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):E2042-9. doi: 10.1073/pnas.1205128109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753491 Free PMC article. - Fig4 deficiency: a newly emerged lysosomal storage disorder?
Martyn C, Li J. Martyn C, et al. Prog Neurobiol. 2013 Feb-Mar;101-102:35-45. doi: 10.1016/j.pneurobio.2012.11.001. Epub 2012 Nov 16. Prog Neurobiol. 2013. PMID: 23165282 Free PMC article. Review. - Roles of PIKfyve in multiple cellular pathways.
Rivero-Ríos P, Weisman LS. Rivero-Ríos P, et al. Curr Opin Cell Biol. 2022 Jun;76:102086. doi: 10.1016/j.ceb.2022.102086. Epub 2022 May 16. Curr Opin Cell Biol. 2022. PMID: 35584589 Free PMC article. Review. - The vacuolar morphology protein VAC14 plays an important role in sexual development in the filamentous ascomycete Sordaria macrospora.
Groth A, Ahlmann S, Werner A, Pöggeler S. Groth A, et al. Curr Genet. 2022 Aug;68(3-4):407-427. doi: 10.1007/s00294-022-01244-0. Epub 2022 Jul 1. Curr Genet. 2022. PMID: 35776170 Free PMC article. - The LAMMER Kinase MoKns1 Regulates Growth, Conidiation and Pathogenicity in Magnaporthe oryzae.
Li L, Zhu XM, Wu JQ, Cao N, Bao JD, Liu XH, Lin FC. Li L, et al. Int J Mol Sci. 2022 Jul 22;23(15):8104. doi: 10.3390/ijms23158104. Int J Mol Sci. 2022. PMID: 35897680 Free PMC article.
References
- Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005;118:2093–2104. - PubMed
- Barth H., Meiling-Wesse K., Epple U. D., Thumm M. Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 2001;508:23–28. - PubMed
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases