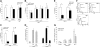Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion - PubMed (original) (raw)
Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion
Haifa Ghandour et al. Blood. 2007.
Abstract
Regulated adhesion of T cells by the integrins LFA-1 (lymphocyte function-associated antigen-1) and VLA-4 (very late antigen-4) is essential for T-cell trafficking. The small GTPase Rap1 is a critical activator of both integrins in murine lymphocytes and T-cell lines. Here we examined the contribution of the Rap1 regulatory pathway in integrin activation in primary CD3(+) human T cells. We demonstrate that inactivation of Rap1 GTPase in human T cells by expression of SPA1 or Rap1GAP blocked stromal cell-derived factor-1alpha (SDF-1alpha)-stimulated LFA-1-ICAM-1 (intercellular adhesion molecule-1) interactions and LFA-1 affinity modulation but unexpectedly did not significantly affect binding of VLA-4 to its ligand VCAM-1 (vascular cell adhesion molecule 1). Importantly, silencing of the Rap1 guanine exchange factor CalDAG-GEFI inhibited SDF-1alpha- and phorbol 12-myristate 13-acetate (PMA)-induced adhesion to ICAM-1 while having no effect on adhesion to VCAM-1. Pharmacologic inhibition of Phospholipase C (PLC) blocked Rap1 activation and inhibited cell adhesion and polarization on ICAM-1 and VCAM-1. Protein kinase C (PKC) inhibition led to enhanced levels of active Rap1 concomitantly with increased T-cell binding to ICAM-1, whereas adhesion to VCAM-1 was reduced. Thus, PLC/CalDAG-GEFI regulation of Rap1 is selectively required for chemokine- and PMA-induced LFA-1 activation in human T cells, whereas alternate PLC- and PKC-dependent mechanisms are involved in the regulation of VLA-4.
Figures
Figure 1
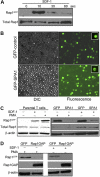
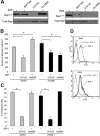
Rap1 activation in human primary T cells. (A) Primary human T cells were stimulated with 100 nM SDF-1α for the indicated times in seconds, and Rap1 (Rap1GTP) was detected by pull-down assay. Total Rap1 is shown as loading control. (B) Transient transfection of T cells with GFP-control (upper) and GFP-SPA1 vectors (lower). Twenty-four hours after transfection, cells were visualized by DIC (left) or fluorescence (right) to evaluate GFP expression and transfection efficiency. Insets show peripheral localization of GFP-SPA1 compared with GFP-control. See “Cell adhesion assay” and “Laminar shear flow assays” for image acquisition information. (C) Analysis of Rap1 activation in parental T cells (left) and GFP-control– or GFP-SPA1–expressing cells (right). Cells were stimulated with 100 nM SDF-1α for 10 seconds or 1μg/mL PMA for 5 minutes, and active Rap1 (Rap1GTP) was determined as in panel A. Cells expressing GFP-SPA1 showed significant reduction in levels of active Rap1 compared with GFP-control-expressing cells. Total Rap1 and β-actin are shown as loading controls. (D) Rap1 activation in T cells expressing GFP-control or Rap1GAP. Cells were stimulated with PMA or SDF-1α as described in panel C, and active Rap1 (Rap1GTP), Rap1GAP levels, and β-actin (loading control) were evaluated. Overexpression of Rap1GAP efficiently reduced levels of active Rap1. Three to 5 independent experiments were done for each panel.
Figure 2
Role of Rap1 GTPase in LFA-1– and VLA-4–mediated T-cell adhesion. (A) Adhesion of GFP-control– or GFP-SPA1–transfected T cells to immobilized ICAM-1 (2.5 μg/mL) or VCAM-1 (2 μg/mL) after treatment with 1 μg/mL PMA or 50 nM Mn2+. Percentage of adherent GFP-positive cells from initial GFP-positive cell input is expressed as mean plus or minus standard error of the mean (SEM). SPA1-GFP-positive cells failed to adhere to ICAM-1 after stimulation with PMA, whereas adhesion to VCAM-1 and Mn2+-induced adhesion to ICAM-1 were unaffected. *P < .001. (B) Effect of VCAM-1 concentration on cell adhesion. T cells were transfected as in panel A and assayed for PMA-induced adhesion to the indicated concentrations of immobilized VCAM-1. Adhesion of cells overexpressing GFP-SPA1 was comparable with GFP-control cells at all doses of VCAM-1 analyzed. (C) T cells transfected with Rap1GAP failed to adhere to immobilized ICAM-1 after PMA stimulation compared with cells transfected with GFP-control alone but were capable of adhering to immobilized VCAM-1 after similar PMA stimulation. *P < .05. (D) Adhesion to VCAM-1 is α4-dependent. T cells were incubated with a functional blocking antibody to VLA-4 (mAb HP2/1), and PMA-induced adhesion to ICAM-1 and VCAM-1 was performed as described in panel A. Blocking α4 had no effect on ICAM-1 binding but completely abolished adhesion to VCAM-1. *P < .001. (E) Effects of inactivation of Rap1 by SPA1 on SDF1-stimulated adhesion to ICAM-1 or VCAM-1 under shear flow conditions. GFP-control or GFP-SPA1-positive T cells were perfused over ICAM-1 or VCAM-1 coimmobilized with SDF-1α at a shear stress rate of 0.75 dyne/cm2. Percentage of adherent GFP-positive cells from total GFP-positive cell input is expressed as the mean plus or minus SEM; *P < .01. T cells overexpressing GFP-SPA1 failed to adhere to ICAM-1 but were able to adhere to VCAM-1 in response to SDF-1α compared with GFP-control cells. (F) Flow cytometry of GFP-control or GFP-SPA1-positive T cells stained with the high-affinity extension reporter mAb KIM127 in the absence (Basal) or presence (top left) of PMA or SDF-1α (top right) or Mn2+ (bottom). GFP-SPA1 cells failed to expose the neoepitope KIM127 in response to both PMA and SDF-1α compared with a substantial induction in GFP-control cells. In contrast, SPA1 cells expressed the KIM127 neoepitope in response to Mn2+. (G) sVCAM-1/Fc binding was determined by flow cytometry after 30 s stimulation with vehicle control, PMA, SDF-1α, or Mn2+. Data are expressed as percentage of events of GFP-positive cells that were positive for APC. Agonist-induced up-regulation of α4 integrin affinity as measured by sVCAM-1/Fc binding was similar in SPA-1 and GFP-control cells. Three to 5 independent experiments were done for each panel.
Figure 3
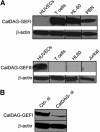
CalDAG-GEFs protein expression. (A) Western blot analysis of human T cells, peripheral blood neutrophils (PBN), human umbilical vain endothelial cells (HUVECs), HL60 promyelocytic cell line, and Jurkat T cells using specific mAb for CalDAG-GEFI (top) and CalDAG-GEFIII (bottom). (B) T cells were transfected with CalDAG-GEFI-specific (CalDAG-si) or control nonsilencing (Ctrl-si) siRNA, and total lysates were immunoblotted with mAb to CalDAG-GEFI. β-actin is shown as loading control. Representative blots are shown. Two to 5 independent experiments were done for each panel. Vertical lines have been inserted to indicate a repositioned gel lane.
Figure 4
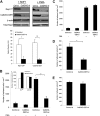
Role of CalDAG-GEFI in LFA-1– and VLA-4–mediated T-cell adhesion. (A) Representative Western blots of Rap1-GTP and CalDAG-GEFI levels in T cells transfected with control or CalDAG-GEFI siRNA and stimulated with 100 nM SDF-1α for 10 seconds (left) or 1μg/mL PMA for 5 minutes (right). β-actin is shown as a loading control. Severely diminished activation of Rap1 was observed in CalDAG-GEFI knock-down cells. Densitometric analysis evaluated the optical density, in arbitrary units, of Rap1-GTP levels after stimulation with SDF-1α in control siRNA and CalDAG-GEFI siRNA cells. CalDAG-GEFI levels in the same samples were also quantified by densitometry. Extent of Rap1 inactivation correlated with CalDAG-GEFI knock-down. *P < .04, **P < .005. (B) PMA-stimulated cell adhesion to immobilized ICAM-1 (2.5 μg/mL) by control or CalDAG-GEFI siRNA-transfected cells shows impaired adhesion in cells deficient in CalDAG-GEFI. *P < .001. Inset, Mn2+ or Ca2+ ionophore (A23187) treatments of T cells transfected with CalDAG-GEFI siRNA resulted in normal adhesion to ICAM-1. (C) Silencing CalDAG-GEFI does not prevent PMA-stimulated T-cell adhesion to immobilized VCAM-1 (2 μg/mL), compared with cells transfected with control siRNA. (D) Effects of CalDAG-GEFI knock-down on chemokine-stimulated LFA-1 adhesion to ICAM-1 under shear flow. T cells transfected with control siRNA or CalDAG-GEFI siRNA were perfused over ICAM-1 coimmobilized with SDF-1α at shear stress rate of 0.75 dyne/cm2. The number of adherent cells is expressed as the mean plus or minus SEM. T cells lacking CalDAG-GEFI show impaired SDF-1α-mediated adhesion to ICAM-1 compared with control cells. *P < .05. (E) T cells, treated as described in panel D, were perfused over VCAM-1 coimmobilized with SDF-1α. Both control siRNA cells and CalDAG-GEFI siRNA cells adhered to VCAM-1. Three to 5 independent experiments were done for each panel.
Figure 5
Role of PLC and PKC in Rap1 activation and stimulated T-cell adhesion. (A) Western blots of Rap1-GTP in T cells pretreated with a PLC inhibitor (10 μM U73122), a calcium chelator (1 μM BAPTA-AM), or a PKC inhibitor (10 μM Gö 6850) for 10 minutes, and then treated with 100 nM SDF-1α for 10 seconds (left) or 1μg/mL PMA for 5 minutes (right). Active Rap1 was detected by pull-down assay. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Inhibition of PLC or PKC impairs SDF1-stimulated adhesion to ICAM-1 or VCAM-1 under shear flow. T cells were pretreated with 10 μM U73211 or 10 μM Gö 6850 for 10 minutes and perfused over ICAM-1 or VCAM-1 coimmobilized with SDF-1α at shear stress rate of 0.75 dyne/cm2. The number of adherent cells is expressed as the mean plus or minus SEM, *indicates P less than .02. (C) The percentage of adherent cells with polarized morphology was determined. Cell polarization was inhibited by the PLC inhibitor (U73122), whereas PKC inhibition (Gö 6850) had no observable effect. Data are the mean plus or minus SEM, *indicates P less than .02. (D) PLC-dependent activation of LFA-1. Flow cytometry of T cells incubated with 10 μM U73211 (left) or 10 μM Gö 6850 (right) for 10 minutes and stained with the high-affinity extension reporter mAb KIM127 in the absence (Basal) or presence of SDF-1α. Inhibition of PLC prevented the exposure of the KIM127 neoepitope in response to SDF-1α, whereas PKC inhibition had no effect. Three to 5 independent experiments were done for each panel.
Figure 6
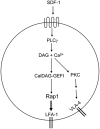
Model of chemokine induced T-cell adhesion through LFA-1 and VLA-4. Chemokine-dependent G-protein-coupled-receptor signaling leads to activation of PLCγ. PLCγ products Ca2+ and DAG activate CalDAG-GEFI. This leads to Rap1 activation, which triggers inside-out activation of LFA-1 to promote T-cell adhesion. SDF-1α activates VLA-4 through PLCγ. PLCγ-generated products may promote PKC activation and subsequent integrin activation through unknown intermediates.
Similar articles
- Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1.
Katagiri K, Shimonaka M, Kinashi T. Katagiri K, et al. J Biol Chem. 2004 Mar 19;279(12):11875-81. doi: 10.1074/jbc.M310717200. Epub 2003 Dec 31. J Biol Chem. 2004. PMID: 14702343 - The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion.
de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. de Bruyn KM, et al. J Biol Chem. 2002 Aug 16;277(33):29468-76. doi: 10.1074/jbc.M204990200. Epub 2002 May 24. J Biol Chem. 2002. PMID: 12171996 - The small GTPase, Rap1, mediates CD31-induced integrin adhesion.
Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. Reedquist KA, et al. J Cell Biol. 2000 Mar 20;148(6):1151-8. doi: 10.1083/jcb.148.6.1151. J Cell Biol. 2000. PMID: 10725328 Free PMC article. - Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases.
Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Yusuf-Makagiansar H, et al. Med Res Rev. 2002 Mar;22(2):146-67. doi: 10.1002/med.10001. Med Res Rev. 2002. PMID: 11857637 Review. - Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL.
Kinashi T, Katagiri K. Kinashi T, et al. Immunol Lett. 2004 Apr 30;93(1):1-5. doi: 10.1016/j.imlet.2004.02.008. Immunol Lett. 2004. PMID: 15134891 Review.
Cited by
- A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model.
Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, Winklmeier S, Zhuo L, Wichmann C, Gerdes LA, Kümpfel T, Dornmair K, Beltrán E, Kerschensteiner M, Kawakami N. Kendirli A, et al. Nat Neurosci. 2023 Oct;26(10):1713-1725. doi: 10.1038/s41593-023-01432-2. Epub 2023 Sep 14. Nat Neurosci. 2023. PMID: 37709997 Free PMC article. - LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation.
Shi H, Shao B. Shi H, et al. Cells. 2023 Apr 12;12(8):1136. doi: 10.3390/cells12081136. Cells. 2023. PMID: 37190045 Free PMC article. Review. - Nexinhib20 Inhibits Neutrophil Adhesion and β2 Integrin Activation by Antagonizing Rac-1-Guanosine 5'-Triphosphate Interaction.
Liu W, Cronin CG, Cao Z, Wang C, Ruan J, Pulikkot S, Hall A, Sun H, Groisman A, Chen Y, Vella AT, Hu L, Liang BT, Fan Z. Liu W, et al. J Immunol. 2022 Oct 15;209(8):1574-1585. doi: 10.4049/jimmunol.2101112. Epub 2022 Sep 7. J Immunol. 2022. PMID: 36165184 Free PMC article. - The Role of RASGRP2 in Vascular Endothelial Cells-A Mini Review.
Takino JI, Miyazaki S, Nagamine K, Hori T. Takino JI, et al. Int J Mol Sci. 2021 Oct 15;22(20):11129. doi: 10.3390/ijms222011129. Int J Mol Sci. 2021. PMID: 34681791 Free PMC article. Review. - Mitofusin-2 regulates leukocyte adhesion and β2 integrin activation.
Liu W, Hsu AY, Wang Y, Lin T, Sun H, Pachter JS, Groisman A, Imperioli M, Yungher FW, Hu L, Wang P, Deng Q, Fan Z. Liu W, et al. J Leukoc Biol. 2022 Apr;111(4):771-791. doi: 10.1002/JLB.1A0720-471R. Epub 2021 Sep 8. J Leukoc Biol. 2022. PMID: 34494308 Free PMC article.
References
- Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. - PubMed
- Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. - PubMed
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous