Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis - PubMed (original) (raw)
Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis
Zhenyuan Song et al. Am J Physiol Gastrointest Liver Physiol. 2007 Oct.
Abstract
Although simple steatosis was originally thought to be a pathologically inert histological change, fat accumulation in the liver may play a critical role not only in disease initiation, but also in the progression to nonalcoholic steatohepatitis and cirrhosis. Therefore, prevention of fat accumulation in the liver may be an effective therapy for multiple stages of nonalcoholic fatty liver disease (NAFLD). Promising beneficial effects of betaine supplementation on human NAFLD have been reported in some pilot clinical studies; however, data related to betaine therapy in NAFLD are limited. In this study, we examined the effects of betaine on fat accumulation in the liver induced by high-sucrose diet and evaluated mechanisms by which betaine could attenuate or prevent hepatic steatosis in this model. Male C57BL/6 mice weighing 20 +/- 0.5 g (means +/- SE) were divided into four groups (8 mice per group) and started on one of four treatments: standard diet (SD), SD+betaine, high-sucrose diet (HS), and HS + betaine. Betaine was supplemented in the drinking water at a concentration of 1% (wt/vol) (anhydrous). Long-term feeding of high-sucrose diet to mice caused significant hepatic steatosis accompanied by markedly increased lipogenic activity. Betaine significantly attenuated hepatic steatosis in this animal model, and this change was associated with increased activation of hepatic AMP-activated protein kinase (AMPK) and attenuated lipogenic capability (enzyme activities and gene expression) in the liver. Our findings are the first to suggest that betaine might serve as a therapeutic tool to attenuate hepatic steatosis by targeting the hepatic AMPK system.
Figures
Fig. 1
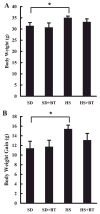
Betaine supplementation had no effect on body weight change in C57BL/6 mice fed high-sucrose diet. A: changes of absolute body weight. C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in drinking water for 16 wk. Data are means ± SD (n = 8), *P < 0.05. SD, standard diet; HS, high-sucrose diet; BT, betaine. B: changes of body weight gain. C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in drinking water (n = 8 mice per group) for 16 wk. Data are means ± SD (n = 8), *P < 0.05.
Fig. 2
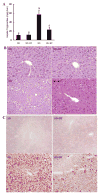
Betaine supplementation attenuated fat accumulation in the liver induced by prolonged high-sucrose feeding. A: hepatic triglyceride content. C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in drinking water for 16 wk. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05). B: formalin-fixed and paraffin-embedded liver sections were stained with hematoxylin and eosin. Livers from SD-fed animals had normal architecture. In contrast, livers from HS-fed animals had obvious fat infiltration with no inflammation or obvious cell death observed. Betaine supplementation groups had significantly less fat accumulation in the liver compared with HS-fed animals. Original magnification ×130. C: flash-frozen liver sections were stained with Oil Red O. SD-fed animals had no obvious fat accumulation in the liver. HS feeding resulted in profound hepatic steatosis, featured by remarkable amount of fat droplet in the liver. Betaine supplementation significantly alleviated hepatic fat accumulation induced by HS diet feeding.
Fig. 3
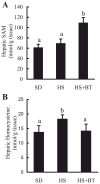
Changes of hepatic methionine metabolism. Male C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in the drinking water for 16 wk. Liver samples were homogenized with 4% metaphosphoric acid and hepatic _S_-adenosylmethionine (SAM) and homocysteine contents were assayed by high-performance liquid chromatography. A: changes of hepatic SAM levels. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05). B: hepatic homocysteine levels. Data are means ± SD (n = 8). Bars with different letters differ significantly (P < 0.05).
Fig. 4
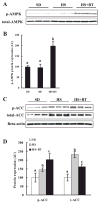
Betaine supplementation resulted in increased phosphorylation (p-) of hepatic AMP-activated protein kinase (AMPK) and inhibited acetyl-CoA carboxylase (ACC) activation. Male C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in the drinking water for 16 wk. Total protein extracts from liver tissues were prepared thereafter. Forty micrograms of protein were subjected to Western blot analysis for AMPK or ACC phosphorylation using antibodies specific for AMPK phosphorylated at Thr172 and ACC phosphorylated at Ser79. A: Western blot assay for AMPK. Betaine supplementation increased phosphorylation of liver AMPK (activation). B: quantification of p-AMPK protein levels in different groups. Levels were normalized to total AMPK. Data are means ± SD (n = 6–7). Bars with different letters differ significantly (P < 0.05). C: Western blot assay for ACC. Betaine supplementation attenuated the increase of ACC protein content induced by high-sucrose feeding and increased phosphorylation of ACC (inhibition) in the liver. D: quantification of p-ACC and total ACC protein levels in different groups. Levels were normalized to actin. Data are means ± SD (n = 6–7). Bars with different letters differ significantly (P < 0.05).
Fig. 5
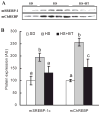
Betaine supplementation suppressed increases of sterol regulatory element-binding protein-1 (SREBP-1) and carbohydrate response element-binding protein (ChREBP) in nuclei induced by high-sucrose feeding. Male C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in the drinking water for 16 wk. Nuclear proteins were isolated from fresh liver tissues thereafter. Forty micrograms of protein were subjected to Western blot analysis for SREBP-1 or ChREBP using specific antibodies. A: prolonged high-sucrose feeding caused significant increase of both SREBP-1 and ChREBP levels in the nuclei, which were suppressed by betaine supplementation. m denotes mature form of the protein (in the nuclei). B: quantification of mSREBP-1c and mChREBP protein levels in different groups. Data are means ± SD (n = 6–7). Bars with different letters differ significantly (P < 0.05).
Fig. 6
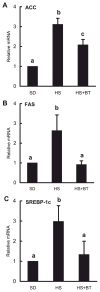
Betaine supplementation suppressed high-sucrose feeding-induced gene expression of ACC (A), fatty acid synthase (FAS; B), and SREBP-1d (C) in the liver. Male C57BL/6 mice were fed with high-sucrose diet in the presence or absence of betaine supplementation (1%) in the drinking water for 16 wk. Total RNA from liver tissues were isolated thereafter and subjected to real-time RT-PCR assay to quantitate mRNAs for ACC, FAS, and SREBP-1c. HS significantly elevated expression of all 3 genes compared with the SD group. Supplementation with betaine suppressed the elevated expression of these genes induced by HS feeding. Data are means ± SD (n = 4). Bars with different letters differ significantly (P < 0.05).
Fig. 7
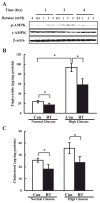
Betaine activated AMPK and reduced lipid accumulation in HepG2 cells. A: betaine activated AMPK in vitro. HepG2 cells were cultured in serum-free DMEM medium overnight and incubated in DMEM containing varying concentrations of betaine for 1, 2, and 4 h, respectively. Total cell extracts were subjected to Western blot analysis with phospho-Thr172 AMPK (p-AMPK) and total AMPKα (t-AMPK) antibodies. Western blots shown represent 3 independent experiments. B and C: betaine lowered the lipid accumulation induced by glucose inclusion in the media in HepG2 cells. HepG2 cells were cultured in serum-free DMEM medium overnight and incubated in DMEM containing either normal (5.5 mM) or high (25 mM) glucose in the absence (Con) or presence of 2 mM betaine for an additional 24 h. Data represent a mean of at least 3 experiments ± SD, *P < 0.05.
Similar articles
- Betaine in ameliorating alcohol-induced hepatic steatosis.
Rehman A, Mehta KJ. Rehman A, et al. Eur J Nutr. 2022 Apr;61(3):1167-1176. doi: 10.1007/s00394-021-02738-2. Epub 2021 Nov 24. Eur J Nutr. 2022. PMID: 34817678 Free PMC article. Review. - Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease.
Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Wang Z, et al. Am J Physiol Gastrointest Liver Physiol. 2010 May;298(5):G634-42. doi: 10.1152/ajpgi.00249.2009. Epub 2010 Mar 4. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20203061 Free PMC article. - Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice.
Hsu WH, Chen TH, Lee BH, Hsu YW, Pan TM. Hsu WH, et al. Food Chem Toxicol. 2014 Feb;64:94-103. doi: 10.1016/j.fct.2013.11.015. Epub 2013 Nov 22. Food Chem Toxicol. 2014. PMID: 24275089 - Involvement of 5'-Activated Protein Kinase (AMPK) in the Effects of Resveratrol on Liver Steatosis.
Trepiana J, Milton-Laskibar I, Gómez-Zorita S, Eseberri I, González M, Fernández-Quintela A, Portillo MP. Trepiana J, et al. Int J Mol Sci. 2018 Nov 5;19(11):3473. doi: 10.3390/ijms19113473. Int J Mol Sci. 2018. PMID: 30400630 Free PMC article. Review.
Cited by
- FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice.
Chen J, Zhou X, Wu W, Wang X, Wang Y. Chen J, et al. J Physiol Biochem. 2015 Sep;71(3):405-13. doi: 10.1007/s13105-015-0420-1. Epub 2015 Jun 16. J Physiol Biochem. 2015. PMID: 26078098 - Regulatory Effects of Functional Soluble Dietary Fiber from Saccharina japonica Byproduct on the Liver of Obese Mice with Type 2 Diabetes Mellitus.
Zhang L, Wang X, He Y, Cao J, Wang K, Lin H, Qu C, Miao J. Zhang L, et al. Mar Drugs. 2022 Jan 21;20(2):91. doi: 10.3390/md20020091. Mar Drugs. 2022. PMID: 35200621 Free PMC article. - Steatosis and steatohepatitis: complex disorders.
Bettermann K, Hohensee T, Haybaeck J. Bettermann K, et al. Int J Mol Sci. 2014 Jun 3;15(6):9924-44. doi: 10.3390/ijms15069924. Int J Mol Sci. 2014. PMID: 24897026 Free PMC article. Review. - An Updated Bio-Behavioral Profile of the Flinders Sensitive Line Rat: Reviewing the Findings of the Past Decade.
Steyn SF. Steyn SF. Pharmacol Res Perspect. 2025 Feb;13(1):e70058. doi: 10.1002/prp2.70058. Pharmacol Res Perspect. 2025. PMID: 39786312 Free PMC article. Review. - Betaine in ameliorating alcohol-induced hepatic steatosis.
Rehman A, Mehta KJ. Rehman A, et al. Eur J Nutr. 2022 Apr;61(3):1167-1176. doi: 10.1007/s00394-021-02738-2. Epub 2021 Nov 24. Eur J Nutr. 2022. PMID: 34817678 Free PMC article. Review.
References
- Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711–2717. - PubMed
- Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17:S186–S190. - PubMed
- Barak AJ, Beckenhauer HC, Badakhsh S, Tuma DJ. The effect of betaine in reversing alcoholic steatosis. Alcohol Clin Exp Res. 1997;21:1100–1102. - PubMed
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
- Caldwell SH, Argo CK, Al-Osaimi AM. Therapy of NAFLD: insulin sensitizing agents. J Clin Gastroenterol. 2006;40:S61–S66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical