Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction - PubMed (original) (raw)
Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction
Zhongqin Zhang et al. Plant Physiol. 2007 Oct.
Abstract
Salicylic acid (SA) plays a central role in plant disease resistance, and emerging evidence indicates that auxin, an essential plant hormone in regulating plant growth and development, is involved in plant disease susceptibility. GH3.5, a member of the GH3 family of early auxin-responsive genes in Arabidopsis (Arabidopsis thaliana), encodes a protein possessing in vitro adenylation activity on both indole-3-acetic acid (IAA) and SA. Here, we show that GH3.5 acts as a bifunctional modulator in both SA and auxin signaling during pathogen infection. Overexpression of the GH3.5 gene in an activation-tagged mutant gh3.5-1D led to elevated accumulation of SA and increased expression of PR-1 in local and systemic tissues in response to avirulent pathogens. In contrast, two T-DNA insertional mutations of GH3.5 partially compromised the systemic acquired resistance associated with diminished PR-1 expression in systemic tissues. The gh3.5-1D mutant also accumulated high levels of free IAA after pathogen infection and impaired different resistance-gene-mediated resistance, which was also observed in the GH3.6 activation-tagged mutant dfl1-D that impacted the auxin pathway, indicating an important role of GH3.5/GH3.6 in disease susceptibility. Furthermore, microarray analysis showed that the SA and auxin pathways were simultaneously augmented in gh3.5-1D after infection with an avirulent pathogen. The SA pathway was amplified by GH3.5 through inducing SA-responsive genes and basal defense components, whereas the auxin pathway was derepressed through up-regulating IAA biosynthesis and down-regulating auxin repressor genes. Taken together, our data reveal novel regulatory functions of GH3.5 in the plant-pathogen interaction.
Figures
Figure 1.
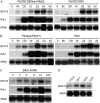
Phenotypic and molecular characterization of gh3.5-1D. A, T-DNA insertion in gh3.5-1D. The large arrow indicates the GH3.5 gene. The four cauliflower mosaic virus 35S enhancer elements are indicated with small arrowheads. B, Four-week-old Col-0, heterozygous (gh3.5-1D+/−), homozygous (gh3.5-1D_−/_−), and representative p35S_∷_GH3.5 transgenic plant. C, Northern-blot analysis (top) of GH3.5 expression and western-blot detection (bottom) of the GH3.5 protein in Col-0, gh3.5-1D+/−, gh3.5-1D_−/_−, and p35S_∷_GH3.5 plants. Rubisco staining was used as a loading control in western blots. The experiments were repeated at least once with similar results.
Figure 2.
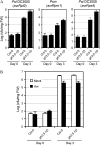
Pathogen growth in leaves of Col-0 and gh3.5-1D plants. A, Growth of avirulent strains Pst DC3000(avrRpt2), Psm(avrRpm1), and Pst DC3000(avrRps4) in Col-0 and gh3.5-1D plants. Plants were infected at a density of 105 cfu/mL. B, Growth of Pst DC3000 in SAR-induced leaves of Col-0 and gh3.5-1D plants. Lower leaves were preinoculated with Psm carrying avrRpm1 at 107 cfu/mL or buffer (mock) 3 d prior to infection with Pst DC3000 at 105 cfu/mL in three systemic leaves. Bacterial titers (A and B) were measured at 0 and 3 dpi. All values are means ±
se
(n = 6). Similar results were observed in three independent experiments.
Figure 3.
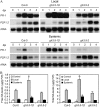
Induction of defense genes and SA accumulation in Col-0, gh3.5-1D, and gh3.5-2 plants. A, Local and systemic induction of PR-1 and PDF1.2 in Col-0, gh3.5-1D, and gh3.5-2 plants. Samples were collected at 0, 1, 2, 3, and 4 dpi with Psm(avrRpm1) at 107 cfu/mL. Experiments were repeated twice with similar results. B, Levels of free and total SA in local and systemic leaves of Col-0, gh3.5-1D, and gh3.5-2 plants after infection with Psm(avrRpm1) at 107 cfu/mL. Leaves were harvested at 0 (control) and 48 hpi. Data are means ±
se
(n = 3). Asterisks indicate significant difference to Col-0 (P < 0.05). Experiments were repeated once with similar results.
Figure 4.
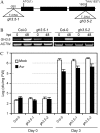
Characterization of GH3.5 loss-of-function mutants gh3.5-1 and gh3.5-2. A, Insertion sites of T-DNA in gh3.5-1 and gh3.5-2. B, RT-PCR detection of the GH3.5 transcript in gh3.5-1 and gh3.5-2 at 0 and 48 hpi with Psm(avrRpm1) at 107 cfu/mL. ACTIN, Loading control for RT-PCR. The experiment was repeated once with similar results. C, Compromised SAR in gh3.5-1 and gh3.5-2 mutants. Growth of Pst DC3000 was measured in plants either mock treated or pretreated with Psm (avrRpm1). All values are mean ±
se
(n = 6). Similar results were obtained in three independent experiments.
Figure 5.
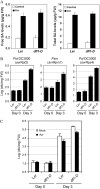
SA levels and pathogen growth in dfl1-D plants. A, Levels of free and total SA in local and systemic leaves of L_er_ and dfl1-D plants after infection with Psm(avrRpm1) at 107 cfu/mL. Leaves were harvested at 0 (control) and 48 hpi. Data are means ±
se
(n = 3). Experiments were repeated once with similar results. B, Growth of Pst DC3000(avrRpt2), Psm(avrRpm1), and Pst DC3000(avrRps4) in L_er_ and dfl1-D plants. Leaves were infected at a density of 105 cfu/mL. C, Growth of Pst DC3000 in L_er_ and dfl1-D plants after preinoculation with Psm(avrRpm1). Data are mean ±
se
(n = 6). Similar results were observed in three independent experiments (B and C).
Figure 6.
Induction pattern of GH3.5 in response to pathogen and SA. A, GH3.5 expression in leaves of Col-0 at different times after infection with Pst DC3000(avrRpt2) and Pst DC3000 at 107 cfu/mL as detected by northern blot. B, Time-course GH3.5 expression in leaves of Col-0 infected with Psm(avrRpm1) and Psm at 107 cfu/mL. Induction of PR-1 was included as a marker for defense activation (A and B). C, Induction of GH3.5 in Col-0 by SA (0.5 m
m
). Samples were harvested at the time points indicated. D, Expression of GH3.5 in cpr1, cpr6, and cpr5 mutants and in Col-0 as the control. All experiments were biologically repeated at least once with similar results.
Figure 7.
Hierarchical clustering of auxin-related genes. Auxin-related genes significantly regulated in gh3.5-1D plants as compared to Col-0 plants. Each column represents a single signal log2 ratio of gh3.5-1D versus Col-0 at 0 (uninoculated) and 48 hpi, and each row represents a different gene. Expression scales (log) are illustrated with colors. Descriptions and accession numbers of the genes are indicated (see also Supplemental Table S4).
Figure 8.
Metabolism changes in gh3.5-1D plants. MAPMAN was used to observe metabolic changes in gh3.5-1D plants either uninoculated or inoculated with Pst DC3000(avrRpt2) at 48 hpi. The average fold change of the three biological replicates is presented as illustrated in the fold change colors in the bottom right of each image (red, repressed; blue, induced).
Figure 9.
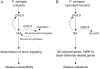
Function model of GH3.5 in Arabidopsis-P. syringae interactions. A, In the compatible interaction, GH3.5 is activated to modulate the auxin pathway resulting in enhanced disease susceptibility through increasing IAA biosynthesis and derepressing auxin signaling. IAA also induces GH3.5 to enlarge those processes. In addition, GH3.5 might also function as an IAA-amido synthetase to regulate IAA homeostasis. B, In the compatible/incompatible interactions, GH3.5 positively modulates the SA pathway to enhance plant defense response through elevating SA biosynthesis, activating SA-induced genes, _WRKY_s, and basal defense-related genes. Feedback regulation of GH3.5 by SA amplifies those effects. In this case, GH3.5 might also synthesize SA-Asp with unknown function during the interactions.
Similar articles
- Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses.
Mutka AM, Fawley S, Tsao T, Kunkel BN. Mutka AM, et al. Plant J. 2013 Jun;74(5):746-54. doi: 10.1111/tpj.12157. Epub 2013 Mar 25. Plant J. 2013. PMID: 23521356 - Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae.
Jagadeeswaran G, Raina S, Acharya BR, Maqbool SB, Mosher SL, Appel HM, Schultz JC, Klessig DF, Raina R. Jagadeeswaran G, et al. Plant J. 2007 Jul;51(2):234-46. doi: 10.1111/j.1365-313X.2007.03130.x. Epub 2007 May 23. Plant J. 2007. PMID: 17521413 - Preference of Arabidopsis thaliana GH3.5 acyl amido synthetase for growth versus defense hormone acyl substrates is dictated by concentration of amino acid substrate aspartate.
Mackelprang R, Okrent RA, Wildermuth MC. Mackelprang R, et al. Phytochemistry. 2017 Nov;143:19-28. doi: 10.1016/j.phytochem.2017.07.001. Epub 2017 Jul 23. Phytochemistry. 2017. PMID: 28743075 - Auxin and plant-microbe interactions.
Spaepen S, Vanderleyden J. Spaepen S, et al. Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a001438. doi: 10.1101/cshperspect.a001438. Cold Spring Harb Perspect Biol. 2011. PMID: 21084388 Free PMC article. Review. - Dissection of salicylic acid-mediated defense signaling networks.
Lu H. Lu H. Plant Signal Behav. 2009 Aug;4(8):713-7. doi: 10.4161/psb.4.8.9173. Epub 2009 Aug 3. Plant Signal Behav. 2009. PMID: 19820324 Free PMC article. Review.
Cited by
- Influence of stress hormones on the auxin homeostasis in Brassica rapa seedlings.
Salopek-Sondi B, Šamec D, Mihaljević S, Smolko A, Pavlović I, Janković I, Ludwig-Müller J. Salopek-Sondi B, et al. Plant Cell Rep. 2013 Jul;32(7):1031-42. doi: 10.1007/s00299-013-1412-7. Epub 2013 Mar 19. Plant Cell Rep. 2013. PMID: 23508255 - Verticillium longisporum infection affects the leaf apoplastic proteome, metabolome, and cell wall properties in Arabidopsis thaliana.
Floerl S, Majcherczyk A, Possienke M, Feussner K, Tappe H, Gatz C, Feussner I, Kües U, Polle A. Floerl S, et al. PLoS One. 2012;7(2):e31435. doi: 10.1371/journal.pone.0031435. Epub 2012 Feb 20. PLoS One. 2012. PMID: 22363647 Free PMC article. - Crystal structure of an indole-3-acetic acid amido synthetase from grapevine involved in auxin homeostasis.
Peat TS, Böttcher C, Newman J, Lucent D, Cowieson N, Davies C. Peat TS, et al. Plant Cell. 2012 Nov;24(11):4525-38. doi: 10.1105/tpc.112.102921. Epub 2012 Nov 6. Plant Cell. 2012. PMID: 23136372 Free PMC article. - High-Throughput Sequencing Reveals Novel microRNAs Involved in the Continuous Flowering Trait of Longan (Dimocarpus longan Lour.).
Waheed S, Liang F, Zhang M, He D, Zeng L. Waheed S, et al. Int J Mol Sci. 2022 Dec 8;23(24):15565. doi: 10.3390/ijms232415565. Int J Mol Sci. 2022. PMID: 36555206 Free PMC article. - Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice.
Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, Wang S. Ding X, et al. Plant Cell. 2008 Jan;20(1):228-40. doi: 10.1105/tpc.107.055657. Epub 2008 Jan 11. Plant Cell. 2008. PMID: 18192436 Free PMC article.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983 - PubMed
- Bartel B, Fink GR (1995) ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268 1745–1748 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials