PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC - PubMed (original) (raw)
PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC
A Cimini et al. PPAR Res. 2007.
Retraction in
- Retracted: PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC.
Ppar Research. Ppar Research. PPAR Res. 2019 Apr 30;2019:5656198. doi: 10.1155/2019/5656198. eCollection 2019. PPAR Res. 2019. PMID: 31182956 Free PMC article.
Abstract
PPAR isotypes are involved in the regulation of cell proliferation, death, and differentiation, with different roles and mechanisms depending on the specific isotype and ligand and on the differentiated, undifferentiated, or transformed status of the cell. Differentiation stimuli are integrated by key transcription factors which regulate specific sets of specialized genes to allow proliferative cells to exit the cell cycle and acquire specialized functions. The main differentiation programs known to be controlled by PPARs both during development and in the adult are placental differentiation, adipogenesis, osteoblast differentiation, skin differentiation, and gut differentiation. PPARs may also be involved in the differentiation of macrophages, brain, and breast. However, their functions in this cell type and organs still awaits further elucidation. PPARs may be involved in cell proliferation and differentiation processes of neural stem cells (NSC). To this aim, in this work the expression of the three PPAR isotypes and RXRs in NSC has been investigated.
Figures
Figure 1
Contrast phase microscopy of neural stem cells growing in neurospheres (a). In (c), BrdU incorporation is shown. Hoechst nuclear staining of the same field is shown in (b). Bar = 40 _μ_m.
Figure 2
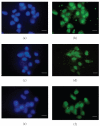
Immunolocalization in S0 neurospheres of nestin (b) and PLP (e). Nuclear staining of the same field is shown in (a) and (d), respectively. Double A2B5/Hoechst immunostaining is shown in (c). Bar = 70 _μ_m.
Figure 2
Immunolocalization in S0 neurospheres of nestin (b) and PLP (e). Nuclear staining of the same field is shown in (a) and (d), respectively. Double A2B5/Hoechst immunostaining is shown in (c). Bar = 70 _μ_m.
Figure 2
Immunolocalization in S0 neurospheres of nestin (b) and PLP (e). Nuclear staining of the same field is shown in (a) and (d), respectively. Double A2B5/Hoechst immunostaining is shown in (c). Bar = 70 _μ_m.
Figure 2
Immunolocalization in S0 neurospheres of nestin (b) and PLP (e). Nuclear staining of the same field is shown in (a) and (d), respectively. Double A2B5/Hoechst immunostaining is shown in (c). Bar = 70 _μ_m.
Figure 2
Immunolocalization in S0 neurospheres of nestin (b) and PLP (e). Nuclear staining of the same field is shown in (a) and (d), respectively. Double A2B5/Hoechst immunostaining is shown in (c). Bar = 70 _μ_m.
Figure 3
PPARs immunolocalization in S0 neurospheres. (b) PPAR_α_, (d) PPAR_β_, (f) PPAR_γ_. Hoechst nuclear staining is shown in (a), (b), and (c), respectively. Bar = 20 _μ_m.
Figure 4
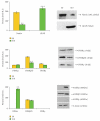
Western blotting and relative densitometric analysis in S0 neurosphere cell lysates. An example of western blotting is shown. Densitometric data are means ± SD of 5 different experiments.
Figure 5
Immunolocalization of nestin, A2B5, and GFAP in S10 neurospheres. In (a), (b), and (c), double immunostaining of nestin/Hoechst, A2B5/Hoechst, and GFAP/Hoechst is shown, respectively. In (d), (e), and (f), the single immunostaining is shown. Bar = 40 _μ_m.
Figure 6
Double immunofluorescence staining for GFAP/PPAR in S10 neurospheres is shown. (a) PPAR_α_, (b) PPAR_β_, (c) PPAR_γ_. Bar = 30 _μ_m.
Figure 7
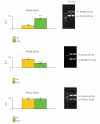
Western blotting and relative densitometric analysis in S10 neurosphere cell lysates. An example of western blotting is shown. Densitometric data are means ± SD of 5 different experiments. * P < .05; ** P < .001.
Figure 8
RT-PCR analysis in S0 and S10 neurospheres. An example of RT-PCR is shown. Densitometric data are means ± SD of 5 different experiments. Semiquantification has been performed against the housekeeping gene _β_-actin. ** P < .001.
Figure 9
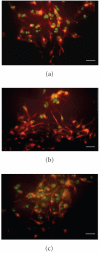
Double oil red/nestin in S0 neurospheres (a) and oil red/GFAP in S10 (b) neurospheres. Bar = 20 _μ_m.
Similar articles
- Retracted: PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC.
Ppar Research. Ppar Research. PPAR Res. 2019 Apr 30;2019:5656198. doi: 10.1155/2019/5656198. eCollection 2019. PPAR Res. 2019. PMID: 31182956 Free PMC article. - Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons.
Cimini A, Benedetti E, Cristiano L, Sebastiani P, D'Amico MA, D'Angelo B, Di Loreto S. Cimini A, et al. Neuroscience. 2005;130(2):325-37. doi: 10.1016/j.neuroscience.2004.09.043. Neuroscience. 2005. PMID: 15664689 - Peroxisome proliferator-activated receptors (PPARs) and related transcription factors in differentiating astrocyte cultures.
Cristiano L, Cimini A, Moreno S, Ragnelli AM, Paola Cerù M. Cristiano L, et al. Neuroscience. 2005;131(3):577-87. doi: 10.1016/j.neuroscience.2004.11.008. Neuroscience. 2005. PMID: 15730864 - Peroxisome proliferator-activated receptors in cutaneous biology.
Kuenzli S, Saurat JH. Kuenzli S, et al. Br J Dermatol. 2003 Aug;149(2):229-36. doi: 10.1046/j.1365-2133.2003.05532.x. Br J Dermatol. 2003. PMID: 12932225 Review. - Peroxisome proliferator-activated receptors, coactivators, and downstream targets.
Qi C, Zhu Y, Reddy JK. Qi C, et al. Cell Biochem Biophys. 2000;32 Spring:187-204. doi: 10.1385/cbb:32:1-3:187. Cell Biochem Biophys. 2000. PMID: 11330046 Review.
Cited by
- Retracted: PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC.
Ppar Research. Ppar Research. PPAR Res. 2019 Apr 30;2019:5656198. doi: 10.1155/2019/5656198. eCollection 2019. PPAR Res. 2019. PMID: 31182956 Free PMC article. - Bezafibrate Upregulates Mitochondrial Biogenesis and Influence Neural Differentiation of Human-Induced Pluripotent Stem Cells.
Augustyniak J, Lenart J, Gaj P, Kolanowska M, Jazdzewski K, Stepien PP, Buzanska L. Augustyniak J, et al. Mol Neurobiol. 2019 Jun;56(6):4346-4363. doi: 10.1007/s12035-018-1368-2. Epub 2018 Oct 13. Mol Neurobiol. 2019. PMID: 30315479 Free PMC article. - PPARs and Energy Metabolism Adaptation during Neurogenesis and Neuronal Maturation.
D'Angelo M, Antonosante A, Castelli V, Catanesi M, Moorthy N, Iannotta D, Cimini A, Benedetti E. D'Angelo M, et al. Int J Mol Sci. 2018 Jun 26;19(7):1869. doi: 10.3390/ijms19071869. Int J Mol Sci. 2018. PMID: 29949869 Free PMC article. Review. - How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus.
Bolijn S, Lucassen PJ. Bolijn S, et al. Brain Plast. 2015 Oct 9;1(1):5-27. doi: 10.3233/BPL-150020. Brain Plast. 2015. PMID: 29765833 Free PMC article. Review. - Energy metabolism in glioblastoma stem cells: PPARα a metabolic adaptor to intratumoral microenvironment.
Fidoamore A, Cristiano L, Laezza C, Galzio R, Benedetti E, Cinque B, Antonosante A, d'Angelo M, Castelli V, Cifone MG, Ippoliti R, Giordano A, Cimini A. Fidoamore A, et al. Oncotarget. 2017 Jul 7;8(65):108430-108450. doi: 10.18632/oncotarget.19086. eCollection 2017 Dec 12. Oncotarget. 2017. PMID: 29312541 Free PMC article.
References
- Nuclear Receptors Nomenclature Committee A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–163. - PubMed
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. - PubMed
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–887. - PubMed
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000;448(2):121–138. - PubMed
- Krey G, Braissant O, L'Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. - PubMed
Publication types
LinkOut - more resources
Full Text Sources