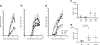Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells - PubMed (original) (raw)
Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells
M Gray et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The maintenance of immune tolerance to apoptotic cells (AC) within an inflammatory milieu is vital to prevent autoimmunity. To investigate this, we administered syngeneic AC i.v. into mice carrying a cohort of ovalbumin (OVA)-specific transgenic T cells (DO11.10) along with OVA peptide and complete Freund's adjuvant, observing a dramatic increase in OVA-specific IL-10 secretion. Activated splenic B cells responded directly to AC, increasing secretion of IL-10, and this programming by AC was key to inducing T cell-derived IL-10. We went on to ask whether AC are able to modulate the course of autoimmune-mediated, chronic inflammation. AC given up to 1 month before the clinical onset of collagen-induced arthritis protected mice from severe joint inflammation and bone destruction. Antigen-specific CD4(+) T cells again secreted significantly more IL-10, associated with a reduced titer of pathogenic anti-collagen II antibodies. Inhibition of IL-10 in vivo reversed the beneficial effects of AC. Passive transfer of B cells from AC-treated mice provided significant protection from arthritis. These data demonstrate that AC exert a profound influence on an adaptive immune response through the generation of CD19(+) regulatory B cells, which in turn are able to influence the cytokine profile of antigen-specific effector T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
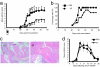
T cells generated in the presence of AC. DO11.10 T cells were transferred into BALB/c mice, immunized with OVA/CFA, and treated with AC or vehicle alone on days 0, 1, and 2. CD4+ T cells harvested on day 7 from the spleens and draining lymph nodes were restimulated with naïve BALB/c splenocytes in the presence of increasing doses of OVA peptide. IL-10 (a), IFN-γ (b), and IL-4 (c) in the supernatants was measured after 72 h. Intracellular cytokine staining was carried out on OVA-specific CD4+KJ126+ T cells (d). Data (mean ± SEM) are representative of three separate experiments with five mice per group for each experiment. **, P < 0.005; ***, P < 0.0007.
Fig. 2.
B cells exposed to AC induce a population of IL-10-secreting OVA-specific T cells. CD11c+ DC and CD19+ B cells from BALB/c mice that had been given CFA/OVA and AC or PBS i.v. 7 days earlier were pulsed with OVA peptide and used as APC to stimulate naïve DO11.10 OVA-specific T cells. Cells were pulsed with tritiated thymidine for the last 12 h of a 72-h culture, and thymidine incorporation was measured (a and b). IL-10 in the supernatants was measured after 72 h in the CD11c+ DC and in the CD19+ B cell cultures (c and d). Data (mean ± SEM) are representative of three separate experiments with five mice per group in each experiment. *, P < 0.03; **, P < 0.009.
Fig. 3.
B cells interact directly with AC, inducing regulation through IL-10. Splenocytes from BALB/c mice were separated into MZ and FO B cell subsets and cultured with naïve DO11.10 T cells in the presence of increasing doses of OVA peptide with or without added AC for 3 days after which supernatants were collected and analyzed for secreted IL-10 (a), IFN-γ (b), and IL-4 (c). Intracellular cytokine staining for IL-10 was performed on purified whole CD19+ B cells (d) and CD4+ T cells (e). To establish the requirement for B cell secretion of IL-10 to induce T cell IL-10 secretion, IL-10 wild-type and IL-10-deficient whole CD19+ B cells were used to stimulate OTII OVA-specific T cells on the C57BL/6 background with and without added AC for 72 h before analysis for secreted IL-10 (f). Data are means ± SEM. *, P < 0.05; **, P < 0.02; ***, P < 0.0001.
Fig. 4.
AC protect mice from CIA. (a) CIA was induced in male DBA-1 mice. The AC group (diamonds) were given an i.v. injection of AC on days 0, 1, and 2, whereas the control group received vehicle only (squares). Clinical disease became apparent by day 20 and increased progressively thereafter (mean ± SEM). *, P < 0.05; **, P < 0.02. (b). The majority of mice given AC at the time of immunization also developed arthritis by day 42, but the severity was significantly less than controls. (c) Ankle joints from mice with CIA at day 56 after immunization with CII/CFA. (ci) Sections through the ankle joint of treated mice show essentially normal joints with no evidence of joint destruction, synovitis, or effusion (hematoxylin and eosin staining). [Magnification: ×100 (×400 for Inset).] (cii) Sections through the ankle joint of control mice show a marked destructive active chronic inflammatory synovitis characterized by synovial hyperplasia, formation of pannus-like tissue, and a fibrinulo-purulent exudate within the joint space (hematoxylin and eosin staining). [Magnification: ×100 (×400 for Inset).] Data are representative of eight separate experiments with between seven and nine mice per group for each experiment. (d) C57BL/6 mice (aged 6–8 weeks) were injected i.v. with AC on days −20, −19, and −18 (AC group, diamonds) whereas the control group received vehicle only (squares). At time −20 days they also received 50 μl of CFA i.d. At time 0 and +2 days they were given 100 μl of K/BxN serum i.p. and then were observed for the onset of clinical arthritis. Data are representative of two separate experiments with six mice per group in each experiment.
Fig. 5.
Coadministration of AC with antigen reduces the level of pathogenic autoantibodies but not CD4+ proliferation. Blood was collected by tail bleed on the days indicated after immunization with CII/CFA. Antibody levels of total IgG (a) and IgG2a (b) were determined and compared with control sera from arthritic mice. On days 11 and 50 after immunization spleens (c) and peripheral lymph nodes (d) were harvested from mice treated with AC (diamonds) and controls treated with vehicle alone (squares). CD4+ T cells were isolated and stimulated with native collagen and syngeneic splenocytes as APC. Cells were pulsed with tritiated thymidine 72 h later and harvested after a further 18 h of culture. Data (mean ± SEM) are representative of four experiments with seven to nine mice per group in each experiment. Differences between groups were ascertained with Student's unpaired t test.
Fig. 6.
Collagen-specific T cells secrete more IL-10 when AC have been administered. (a) Fourteen days after immunization with CII/CFA spleens and peripheral lymph nodes were harvested, and CD4+ T cells were isolated and restimulated with native collagen and syngeneic splenocytes. After 72 h of culture supernatants were tested for IL-10 (a) and IFN-γ (b) cytokine content. (c) Mice were given a blocking anti-IL-10 monoclonal antibody (SXC-1) by i.p. injection at the time of immunization with CII/CFA and AC (open diamonds) or with CII/CFA and PBS control (open squares) and weekly thereafter for 14 days. As indicated they were also given AC (filled diamonds) or vehicle only (filled squares) on days 0, 1, and 2 after immunization. (d) IgG2a levels from arthritic mice were determined from each group. Differences between groups were ascertained with Student's unpaired t test. Data are means ± SEM. *, P < 0.05.
Fig. 7.
Passive transfer of protection from CIA using splenocyte-derived populations. Single-cell suspensions of whole splenocyte populations from AC and CII/CFA or PBS and CII/CFA-immunized mice were isolated from mice on day 21. Twenty million were injected i.v. into naïve mice on day 0, which were then immunized with CII/CFA (a). CD4+ T cells (b) or CD19+ B cells (d) from AC- or PBS-treated mice were injected i.v. into naïve DBA1 mice before immunization with CII/CFA. Tail bleed was taken on the days indicated, and CII-specific IgG2a levels were measured from mice given CD4+ T cells (c) or CD19+ B cells (e). Data are means ± SEM. *, P < 0.05.
Similar articles
- Ex vivo activated OVA specific and non-specific CD4+CD25+ regulatory T cells exhibit comparable suppression to OVA mediated T cell responses.
Li J, Bracht M, Shang X, Radewonuk J, Emmell E, Griswold DE, Li L. Li J, et al. Cell Immunol. 2006 Jun;241(2):75-84. doi: 10.1016/j.cellimm.2006.08.003. Epub 2006 Sep 27. Cell Immunol. 2006. PMID: 17010326 - In vivo splenic CD11c cells downregulate CD4 T-cell response thereby decreasing systemic immunity to gene-modified tumour cell vaccine.
Cayeux S, Bukarica B, Buschow C, Charo J, Bunse M, Dörken B, Blankenstein T. Cayeux S, et al. Gene Ther. 2007 Oct;14(20):1481-91. doi: 10.1038/sj.gt.3303003. Epub 2007 Aug 16. Gene Ther. 2007. PMID: 17700709 - T cell tolerance.
Marrack P, Kappler J. Marrack P, et al. Semin Immunol. 1990 Jan;2(1):45-9. Semin Immunol. 1990. PMID: 2151797 Review. - Role of yoghurt in the prevention of colon cancer.
Perdigón G, de Moreno de LeBlanc A, Valdez J, Rachid M. Perdigón G, et al. Eur J Clin Nutr. 2002 Aug;56 Suppl 3:S65-8. doi: 10.1038/sj.ejcn.1601490. Eur J Clin Nutr. 2002. PMID: 12142967 Review.
Cited by
- Apoptotic Donor Cells in Transplantation.
Husain I, Luo X. Husain I, et al. Front Immunol. 2021 Feb 25;12:626840. doi: 10.3389/fimmu.2021.626840. eCollection 2021. Front Immunol. 2021. PMID: 33717145 Free PMC article. Review. - Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells.
Kubo M, Motomura Y. Kubo M, et al. Front Immunol. 2012 Aug 30;3:275. doi: 10.3389/fimmu.2012.00275. eCollection 2012. Front Immunol. 2012. PMID: 22969768 Free PMC article. - Regulatory B cells in autoimmune diseases and mucosal immune homeostasis.
Li X, Braun J, Wei B. Li X, et al. Autoimmunity. 2011 Feb;44(1):58-68. doi: 10.3109/08916931003782189. Epub 2010 Aug 11. Autoimmunity. 2011. PMID: 20701454 Free PMC article. - Natural regulatory plasma cells.
Fillatreau S. Fillatreau S. Curr Opin Immunol. 2018 Dec;55:62-66. doi: 10.1016/j.coi.2018.09.012. Epub 2018 Oct 4. Curr Opin Immunol. 2018. PMID: 30292126 Free PMC article. Review. - Colitis immunoregulation by CD8+ T cell requires T cell cytotoxicity and B cell peptide antigen presentation.
McPherson M, Wei B, Turovskaya O, Fujiwara D, Brewer S, Braun J. McPherson M, et al. Am J Physiol Gastrointest Liver Physiol. 2008 Sep;295(3):G485-92. doi: 10.1152/ajpgi.90221.2008. Epub 2008 Jul 10. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18617557 Free PMC article.
References
- Savill J, Fadok V. Nature. 2000;407:784–788. - PubMed
- Savill J, Dransfield I, Gregory C, Haslett C. Nat Rev Immunol. 2002;2:965–975. - PubMed
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. - PubMed
- Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. J Immunol. 2002;168:1627–1635. - PubMed
- Gallucci S, Lolkema M, Matzinger P. Nat Med. 1999;5:1249–1255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials