Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle - PubMed (original) (raw)
Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle
Kaiwen Kam et al. J Neurosci. 2007.
Abstract
The glutamate-glutamine cycle is thought to be integral in continuously replenishing the neurotransmitter pool of glutamate. Inhibiting glial transfer of glutamine to neurons leads to rapid impairment in physiological and behavioral function; however, the degree to which excitatory synaptic transmission relies on the normal operation of this cycle is unknown. In slices and cultured neurons from rat hippocampus, we enhanced the transfer of glutamine to neurons, a fundamental step in this cycle, by adding exogenous glutamine. Although raising glutamine augments synaptic transmission by increasing vesicular glutamate, access to this synthetic pathway by exogenously applied glutamine to neurons is delayed and slow, challenging mechanisms linking the rapid effects of pharmacological inhibitors to decreased vesicular glutamate. We find that pharmacological inhibitors of glutamine synthetase or system A transporters cause an acute depression of basal synaptic transmission that is rapidly reversible, which is unlikely to be attributable to the rapid loss of vesicular glutamate. Furthermore, release of vesicular glutamate remains robust even during the prolonged removal of glutamine from pure neuronal cultures. We conclude that neurons have the capacity to store or produce glutamate for long periods of time, independently of glia and the glutamate-glutamine cycle.
Figures
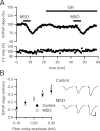
Figure 1.
MSO causes an acute decrease in synaptic transmission that appears to be independent of glutamate–glutamine cycle inhibition. A, fEPSPs recorded in stratum radiatum of CA1 in rat hippocampal slices (n = 9) are inhibited by MSO (10 m
m
); however, glutamine (Gln; 4 m
m
) does not prevent the effect. B, Input–output relationships in slices incubated in MSO (40 m
m
; n = 11) or control solution (n = 9) for >4 h are not significantly different (p > 0.05 for all fiber volley amplitudes). Representative traces at three fiber volley amplitudes are shown to the right for a control slice (top) and a MSO incubated slice (bottom). FV, Fiber volley. Calibration: 0.5 mV, 10 ms.
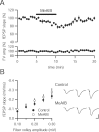
Figure 2.
MeAIB produces a rapidly reversible acute depression of synaptic transmission. A, Depression of fEPSPs recorded in stratum radiatum of CA1 is observed after MeAIB (25 m
m
) application (n = 7). B, Incubation in MeAIB (25 m
m
) for more than 4 h does not change the input–output curve (control, n = 16; MeAIB, n = 14; p > 0.05 for all fiber volley amplitudes). Representative traces in control slices (top) and MeAIB-incubated slices (bottom) are shown to the right. FV, Fiber volley. Calibration: 0.5 mV, 10 ms.
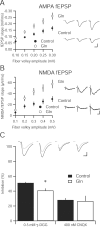
Figure 3.
Increasing extracellular glutamine enhances synaptic transmission and vesicular glutamate content in hippocampal slice. A, Input–output relationship of the AMPA-mediated fEPSPs in hippocampal slices incubated in 4 m
m
glutamine (n = 16) compared with a 4 m
m
sucrose osmotic control (n = 15) are statistically greater at all fiber volley amplitudes (p < 0.05). Sample fEPSPs at different fiber volley amplitudes from each group are shown to the right. Calibration: 0.5 mV, 10 ms. B, Input–output curve of NMDA-mediated fEPSP in 4 m
m
glutamine-incubated slices (n = 13) is significantly enhanced over control slices (n = 12). Representative traces from three fiber volley amplitudes are shown to the right for glutamine-incubated (top) and control (bottom) slices. Calibration: 0.1 mV, 10 ms. C, Inhibition of evoked EPSCs by the low-affinity AMPA receptor antagonist γ-DGG (0.5 m
m
) is reduced in 4 m
m
glutamine-incubated slices (n = 12) relative to control (n = 10; * p < 0.05). However, the high-affinity antagonist CNQX (400 n
m
) produces similar inhibition in both control (n = 7) and glutamine-incubated slices (n = 6; p > 0.05). Inset, Sample traces before (black traces) and after (gray traces) γ-DGG and CNQX application. Calibration: 25 pA, 10 ms.
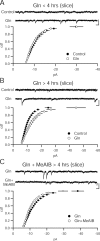
Figure 4.
Prolonged incubation in glutamine increases quantal amplitude through a system A-dependent mechanism. A, Quantal amplitude does not change in slices incubated in glutamine (Gln; 4 m
m
) for <4 h. Cumulative distribution function of amplitudes are not different between glutamine (_n_ = 7) and control slices (_n_ = 9; _p_ > 0.05, Kolmogorov–Smirnov test). Sample recordings from control (top) and glutamine-incubated (bottom) cells. Calibration: 10 pA, 100 ms. B, After more than 4 h of glutamine incubation, quantal amplitude is increased. Cumulative distribution function of amplitudes are significantly increased in glutamine (n = 8) slices over control slices (n = 7; p < 0.05, Kolmogorov–Smirnov test). Sample recordings from control (top) and glutamine-incubated (bottom) cells are shown. Calibration: 10 pA, 100 ms. C, Glutamine enhancement relies on system A transporters. Cumulative distribution function of amplitudes is reduced in slices incubated in glutamine and MeAIB (n = 18) relative to slices incubated in glutamine alone (n = 18; p < 0.05, Kolmogorov–Smirnov test). Sample recordings from glutamine-incubated (top) and glutamine-incubated (bottom) cells. Calibration: 10 pA, 100 ms.
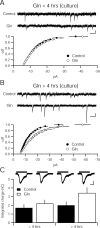
Figure 5.
Glutamine enhances quantal amplitude in dissociated hippocampal culture with the same time course as in hippocampal slices. A, Cumulative distribution function of amplitudes is not different between 4 m
m
glutamine (Gln)-incubated (n = 18) and control cultures (n = 20; p > 0.05, Kolmogorov–Smirnov test). Sample recordings from control (top) and glutamine-incubated (bottom) cells. Calibration: 10 pA, 100 ms. B, Cumulative distribution function of amplitudes is different between 4 m
m
glutamine-incubated (n = 15) and control cultures (n = 14; p < 0.05, Kolmogorov–Smirnov test). Sample recordings from control (top) and glutamine-incubated (bottom) cells. Calibration: 10 pA, 100 ms. C, Current evoked by sucrose (500 m
m
) does not differ between cultures incubated in 4 m
m
glutamine (<4 h, _n_ = 19; >4 h, n = 16) relative to control coverslips (<4 h, _n_ = 16; >4 h, n = 16; p > 0.05). Inset, Representative traces show sucrose-evoked currents. Black bars indicate application of 500 m
m
sucrose. Calibration: 400 pA, 1 s.
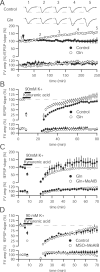
Figure 6.
Glutamine enhancement of synaptic transmission is activity-dependent. A, Time course of glutamine enhancement shows that population activity does not increase appreciably until 1 h after application. Sample traces in control (top) and 4 m
m
glutamine (Gln)-incubated (bottom) slices at baseline, 1, 2, 3, and 4 h after glutamine application. Calibration: 0.5 mV, 10 ms. B, Application of hyperkalemic solution results in an immediate increase in fEPSP slope in the presence of 4 m
m
glutamine (n = 11) relative to control (n = 6) and produces an enhancement over baseline after 60 min. C, Immediate enhancement by 4 m
m
glutamine (n = 7) after hyperkalemic stimulation is lessened by addition of 25 m
m
MeAIB (n = 8), whereas the persistent enhancement after 60 min is abolished. D, Recovery of synaptic transmission after intense hyperkalemic stimulation does not differ in the presence of 40 m
m
MSO and 25 m
m
MeAIB (n = 7) relative to control (n = 5) slices.
Similar articles
- Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons.
Waagepetersen HS, Qu H, Sonnewald U, Shimamoto K, Schousboe A. Waagepetersen HS, et al. Neurochem Int. 2005 Jul;47(1-2):92-102. doi: 10.1016/j.neuint.2005.04.012. Neurochem Int. 2005. PMID: 15921825 - Role of glutamate transporters in corticostriatal synaptic transmission.
Beurrier C, Bonvento G, Kerkerian-Le Goff L, Gubellini P. Beurrier C, et al. Neuroscience. 2009 Feb 18;158(4):1608-15. doi: 10.1016/j.neuroscience.2008.11.018. Epub 2008 Nov 17. Neuroscience. 2009. PMID: 19063944 - Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1.
Liang SL, Carlson GC, Coulter DA. Liang SL, et al. J Neurosci. 2006 Aug 16;26(33):8537-48. doi: 10.1523/JNEUROSCI.0329-06.2006. J Neurosci. 2006. PMID: 16914680 Free PMC article. - Maintaining the presynaptic glutamate supply for excitatory neurotransmission.
Marx MC, Billups D, Billups B. Marx MC, et al. J Neurosci Res. 2015 Jul;93(7):1031-44. doi: 10.1002/jnr.23561. Epub 2015 Feb 3. J Neurosci Res. 2015. PMID: 25648608 Review. - Structure-Function Relationship of Transporters in the Glutamate-Glutamine Cycle of the Central Nervous System.
Hayashi MK. Hayashi MK. Int J Mol Sci. 2018 Apr 12;19(4):1177. doi: 10.3390/ijms19041177. Int J Mol Sci. 2018. PMID: 29649168 Free PMC article. Review.
Cited by
- Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease.
Takahashi K, Foster JB, Lin CL. Takahashi K, et al. Cell Mol Life Sci. 2015 Sep;72(18):3489-506. doi: 10.1007/s00018-015-1937-8. Epub 2015 Jun 2. Cell Mol Life Sci. 2015. PMID: 26033496 Free PMC article. Review. - Vesicular and plasma membrane transporters for neurotransmitters.
Blakely RD, Edwards RH. Blakely RD, et al. Cold Spring Harb Perspect Biol. 2012 Feb 1;4(2):a005595. doi: 10.1101/cshperspect.a005595. Cold Spring Harb Perspect Biol. 2012. PMID: 22199021 Free PMC article. Review. - Good housekeeping.
Overstreet-Wadiche L, Wadiche JI. Overstreet-Wadiche L, et al. Neuron. 2014 Feb 19;81(4):715-7. doi: 10.1016/j.neuron.2014.02.004. Neuron. 2014. PMID: 24559665 Free PMC article. - LY354740 Reduces Extracellular Glutamate Concentration, Inhibits Phosphorylation of Fyn/NMDARs, and Expression of PLK2/pS129 α-Synuclein in Mice Treated With Acute or Sub-Acute MPTP.
Tan Y, Xu Y, Cheng C, Zheng C, Zeng W, Wang J, Zhang X, Yang X, Wang J, Yang X, Nie S, Cao X. Tan Y, et al. Front Pharmacol. 2020 Feb 28;11:183. doi: 10.3389/fphar.2020.00183. eCollection 2020. Front Pharmacol. 2020. PMID: 32180729 Free PMC article. - Synaptic Vesicle Protein NTT4/XT1 (SLC6A17) Catalyzes Na+-coupled Neutral Amino Acid Transport.
Zaia KA, Reimer RJ. Zaia KA, et al. J Biol Chem. 2009 Mar 27;284(13):8439-48. doi: 10.1074/jbc.M806407200. Epub 2009 Jan 15. J Biol Chem. 2009. PMID: 19147495 Free PMC article.
References
- Albrecht J, Norenberg MD. L-methionine-DL-sulfoximine induces massive efflux of glutamine from cortical astrocytes in primary culture. Eur J Pharmacol. 1990;182:587–589. - PubMed
- Armano S, Coco S, Bacci A, Pravettoni E, Schenk U, Verderio C, Varoqui H, Erickson JD, Matteoli M. Localization and functional relevance of system a neutral amino acid transporters in cultured hippocampal neurons. J Biol Chem. 2002;277:10467–10473. - PubMed
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. - PubMed
- Barnett NL, Pow DV, Robinson SR. Inhibition of Muller cell glutamine synthetase rapidly impairs the retinal response to light. Glia. 2000;30:64–73. - PubMed
- Benjamin AM, Quastel JH. Metabolism of amino acids and ammonia in rat brain cortex slices in vitro: a possible role of ammonia in brain function. J Neurochem. 1975;25:197–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources