A genetic screen identifies novel polycomb group genes in Drosophila - PubMed (original) (raw)
A genetic screen identifies novel polycomb group genes in Drosophila
Andrés Gaytán de Ayala Alonso et al. Genetics. 2007 Aug.
Abstract
Polycomb group (PcG) genes encode evolutionarily conserved transcriptional repressors that are required for the long-term silencing of particular developmental control genes in animals and plants. PcG genes were first identified in Drosophila as regulators that keep HOX genes inactive in cells where these genes must remain silent during development. Here, we report the results of a genetic screen aimed at isolating novel PcG mutants in Drosophila. In an EMS mutagenesis, we isolated 82 mutants that show Polycomb-like phenotypes in clones in the adult epidermis and misexpression of the HOX gene Ubx in clones in the imaginal wing disc. Analysis of these mutants revealed that we isolated multiple new alleles in most of the already- known PcG genes. In addition, we isolated multiple mutant alleles in each of ten different genes that previously had not been known to function in PcG repression. We show that the newly identified PcG gene calypso is required for the long-term repression of multiple HOX genes in embryos and larvae. In addition, our studies reveal that the Kto/Med12 and Skd/Med13 subunits of the Med12.Med13.Cdk8.CycC repressor subcomplex of Mediator are needed for repression of the HOX gene Ubx. The results of the mutant screen reported here suggest that the majority of nonredundant Drosophila genes with strong classic PcG phenotypes have been identified.
Figures
Figure 1.—
Screening procedure for novel PcG mutants. The crossing scheme for the mutagenesis on chromosome 3 is shown as an example. EMS-fed F0 males homozygous for FRT2A FRT82B were crossed to vgBE-Gal4 UAS-flp; FRT2A FRT82B y+ “tester” females, and their F1 progeny was screened for appearance of the PcG syndrome in the wing and thorax. Candidate F1 mutants were retested using the same crossing procedure. The mutagenized chromosome was isolated from F2 animals that showed the PcG syndrome, and balanced strains were established. EMS-induced mutation is represented with an asterisk (*). The same basic procedure with appropriate FRT chromosomes was used to isolate mutations on chromosomes 1 and 2 (see
materials and methods
for detailed strain genotypes).
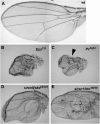
Figure 2.—
PcG syndrome in wings of adult flies with clones of PcG mutant cells. (A) Wing of a wild-type adult. (B–E) Wings from F2 adults isolated in the screen (see Figure 1). The animals were heterozygous for the indicated mutation and contained clones of homozygous mutant cells in the wing, induced by FLP recombinase expressed under the control of vgBE-Gal4 UAS-flp. Wings were photographed at the same magnification. Note the overall reduction of wing size, indicating partial transformation into haltere; blisters and necrotic tissue are observed in the wing blade, possibly reflecting sorting out of clone cells from the surrounding wild-type tissue. (C) Part of the anterior wing margin is transformed into posterior wing margin (arrowhead). Other phenotypes such as pattern duplications are visible as well, but these were not reliable indicators of PcG syndrome (see text). In the case of the two class I mutants E(z)731 (B) and Pc33A3 (C), the PcG syndrome is more severe than in the class II mutants siren9/skd32A24 (D) and siren1/kto32E16 (E).
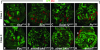
Figure 3.—
Misexpression of the HOX gene Ubx in PcG mutant clones in the wing imaginal disc. Wing imaginal discs with clones of cells that are homozygous for the indicated mutant allele were stained with an antibody against the Ubx protein (red); clones of mutant cells are marked by the absence of GFP protein (green). (A and E) Clones were induced 96 hr before analysis by heat-shock-induced expression of FLP recombinase. (B–D and F–H) The clones were induced by FLP recombinase expressed from the vgBE-Gal4 UAS-flp driver; the clones were thus induced at different time points during larval development and, consequently, the clone size varies considerably. Ubx is normally not expressed in the wing imaginal disc, but it is strongly misexpressed in a large fraction of clones in class I mutants (A–D). In class II mutants (E–H), misexpression of Ubx is observed only in a few rare clones and the levels of Ubx signal in these clones is consistently lower than in class I mutant clones. The asterisk (*) in H marks the normal wild-type expression of Ubx in the wing trachea that was present in this preparation. All discs are oriented with the anterior compartment to the left.
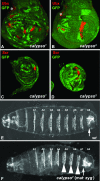
Figure 4.—
The class I mutant calypso shows classic PcG phenotypes. Wing (A and B) and second leg discs (C and D) with calypso1 (A and C) or calypso2 (B and D) mutant clones were stained with antibodies against Ubx (A and B) or Scr (C and D) protein (red). In all cases, clones of calypso mutant cells are marked by the absence of GFP protein (green). Ubx is normally not expressed in the wing imaginal disc and Scr is normally not expressed in the second leg imaginal disc but strong misexpression of Ubx and Scr is detected in calypso1 and calypso2 mutant clones. An asterisk (*) in A and B marks normal Ubx expression in the trachea. (E and F) Ventral views of cuticles of a wild-type embryo (E) and a calypso2 homozygous embryo derived from a calypso2 germ-line clone (F). In the calypso2 mutant embryo, abdominal segments A5–A7 (arrowheads) are homeotically transformed, resembling the eighth abdominal segment (arrow) due to the lack of maternal and zygotic (mat− zyg−) wild-type Calypso protein.
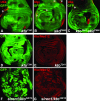
Figure 5.—
Analysis of kto and skd mutant clones in the wing imaginal disc. (A–C) Wing discs with clones of cells homozygous for ktoT241 (A), skdT606 (B), or double homozygous for ktoT241 and skdT606 (C) were stained with antibodies against Ubx protein (red). In all cases, clones of mutant cells were marked by the absence of GFP protein (green). Note that in all cases Ubx is misexpressed only in a fraction of clone cells, but that this phenotype is comparable to that of siren9/skd32A24 or siren1/kto32E16 mutant clones shown in Figure 3, F and G, respectively. Asterisks (*) in A–C mark normal Ubx expression in the trachea and the peripordial membrane. (D–G) Wing discs with ktoT241 (D and E) or siren1/kto32E16 (F and G) mutant clones stained with antibodies against Kto/Med12 protein (red). Clones of mutant cells were marked by the absence of GFP protein (green). (D and E) Clones were induced 96 hr before analysis by heat-shock-induced expression of FLP recombinase. (F and G) The clones were induced by FLP recombinase expressed from the vgBE-Gal4 UAS-flp driver; the clones were thus induced at different time points during larval development and, consequently, the clone sizes vary considerably. Note the lack of Kto/Med12 antibody signal in both ktoT241 and siren1/kto32E16 mutant clones. Loss of the Kto/Med12 epitope is also observed in clones of other siren1/kto mutants (data not shown). All discs are oriented with the anterior compartment to the left.
Similar articles
- Polycomb group genes are required for neural stem cell survival in postembryonic neurogenesis of Drosophila.
Bello B, Holbro N, Reichert H. Bello B, et al. Development. 2007 Mar;134(6):1091-9. doi: 10.1242/dev.02793. Epub 2007 Feb 7. Development. 2007. PMID: 17287254 - Effects of Polycomb group mutations on the expression of Ultrabithorax in the Drosophila visceral mesoderm.
Choi SH, Oh CT, Kim SH, Kim YT, Jeon SH. Choi SH, et al. Mol Cells. 2000 Apr 30;10(2):156-61. doi: 10.1007/s10059-000-0156-8. Mol Cells. 2000. PMID: 10850656 - Comparative analysis of chromatin binding by Sex Comb on Midleg (SCM) and other polycomb group repressors at a Drosophila Hox gene.
Wang L, Jahren N, Miller EL, Ketel CS, Mallin DR, Simon JA. Wang L, et al. Mol Cell Biol. 2010 Jun;30(11):2584-93. doi: 10.1128/MCB.01451-09. Epub 2010 Mar 29. Mol Cell Biol. 2010. PMID: 20351181 Free PMC article. - [Maintenance of cellular memory by Polycomb group genes].
Netter S, Boivin A. Netter S, et al. C R Acad Sci III. 2001 Jul;324(7):577-88. doi: 10.1016/s0764-4469(01)01329-4. C R Acad Sci III. 2001. PMID: 11475999 Review. French. - Genesis versus epigenesis: the odd jobs of the Polycomb group of genes.
Santamaría P. Santamaría P. Int J Dev Biol. 1998;42(3):463-9. Int J Dev Biol. 1998. PMID: 9654032 Review.
Cited by
- Kinetic Characterization of ASXL1/2-Mediated Allosteric Regulation of the BAP1 Deubiquitinase.
Peng H, Cassel J, McCracken DS, Prokop JW, Sementino E, Cheung M, Collop PR, Polo A, Joshi S, Mandell JP, Ayyanathan K, Hinds D, Malkowicz SB, Harbour JW, Bowcock AM, Salvino J, Kennedy EJ, Testa JR, Rauscher FJ 3rd. Peng H, et al. Mol Cancer Res. 2021 Jul;19(7):1099-1112. doi: 10.1158/1541-7786.MCR-20-0080. Epub 2021 Mar 17. Mol Cancer Res. 2021. PMID: 33731362 Free PMC article. - Drosophila Cyclin G and epigenetic maintenance of gene expression during development.
Dupont CA, Dardalhon-Cuménal D, Kyba M, Brock HW, Randsholt NB, Peronnet F. Dupont CA, et al. Epigenetics Chromatin. 2015 May 7;8:18. doi: 10.1186/s13072-015-0008-6. eCollection 2015. Epigenetics Chromatin. 2015. PMID: 25995770 Free PMC article. - Synergy between Variant PRC1 Complexes Defines Polycomb-Mediated Gene Repression.
Fursova NA, Blackledge NP, Nakayama M, Ito S, Koseki Y, Farcas AM, King HW, Koseki H, Klose RJ. Fursova NA, et al. Mol Cell. 2019 Jun 6;74(5):1020-1036.e8. doi: 10.1016/j.molcel.2019.03.024. Epub 2019 Apr 24. Mol Cell. 2019. PMID: 31029541 Free PMC article. - Loss of polycomb repressive complex 1 activity and chromosomal instability drive uveal melanoma progression.
Bakhoum MF, Francis JH, Agustinus A, Earlie EM, Di Bona M, Abramson DH, Duran M, Masilionis I, Molina E, Shoushtari AN, Goldbaum MH, Mischel PS, Bakhoum SF, Laughney AM. Bakhoum MF, et al. Nat Commun. 2021 Sep 13;12(1):5402. doi: 10.1038/s41467-021-25529-z. Nat Commun. 2021. PMID: 34518527 Free PMC article. - De novo dominant ASXL3 mutations alter H2A deubiquitination and transcription in Bainbridge-Ropers syndrome.
Srivastava A, Ritesh KC, Tsan YC, Liao R, Su F, Cao X, Hannibal MC, Keegan CE, Chinnaiyan AM, Martin DM, Bielas SL. Srivastava A, et al. Hum Mol Genet. 2016 Feb 1;25(3):597-608. doi: 10.1093/hmg/ddv499. Epub 2015 Dec 8. Hum Mol Genet. 2016. PMID: 26647312 Free PMC article.
References
- Beachy, P. A., S. L. Helfand and D. S. Hogness, 1985. Segmental distribution of bithorax complex proteins during Drosophila development. Nature 313: 545–551. - PubMed
- Beuchle, D., G. Struhl and J. Müller, 2001. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development 128: 993–1004. - PubMed
- Birve, A., A. K. Sengupta, D. Beuchle, J. Larsson, J. A. Kennison et al., 2001. Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development 128: 3371–3379. - PubMed
- Björklund, S., and C. M. Gustafsson, 2005. The yeast Mediator complex and its regulation. Trends Biochem. Sci. 30: 240–244. - PubMed
- Borggrefe, T., R. Davis, H. Erdjument-Bromage, P. Tempst and R. D. Kornberg, 2002. A complex of the Srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 277: 44202–44207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases