Structures of MART-126/27-35 Peptide/HLA-A2 complexes reveal a remarkable disconnect between antigen structural homology and T cell recognition - PubMed (original) (raw)
Structures of MART-126/27-35 Peptide/HLA-A2 complexes reveal a remarkable disconnect between antigen structural homology and T cell recognition
Oleg Y Borbulevych et al. J Mol Biol. 2007.
Abstract
Small structural changes in peptides presented by major histocompatibility complex (MHC) molecules often result in large changes in immunogenicity, supporting the notion that T cell receptors are exquisitely sensitive to antigen structure. Yet there are striking examples of TCR recognition of structurally dissimilar ligands. The resulting unpredictability of how T cells will respond to different or modified antigens impacts both our understanding of the physical bases for TCR specificity as well as efforts to engineer peptides for immunomodulation. In cancer immunotherapy, epitopes and variants derived from the MART-1/Melan-A protein are widely used as clinical vaccines. Two overlapping epitopes spanning amino acid residues 26 through 35 are of particular interest: numerous clinical studies have been performed using variants of the MART-1 26-35 decamer, although only the 27-35 nonamer has been found on the surface of targeted melanoma cells. Here, we show that the 26-35 and 27-35 peptides adopt strikingly different conformations when bound to HLA-A2. Nevertheless, clonally distinct MART-1(26/27-35)-reactive T cells show broad cross-reactivity towards these ligands. Simultaneously, however, many of the cross-reactive T cells remain unable to recognize anchor-modified variants with very subtle structural differences. These dichotomous observations challenge our thinking about how structural information on unligated peptide/MHC complexes should be best used when addressing questions of TCR specificity. Our findings also indicate that caution is warranted in the design of immunotherapeutics based on the MART-1 26/27-35 epitopes, as neither cross-reactivity nor selectivity is predictable based on the analysis of the structures alone.
Figures
Figure 1
MART-126/27–35-based peptides adopt one of two general conformations in the HLA-A2 peptide-binding groove. (a) Superimposition of the native AAG nonamer and the P2-modified ALG nonamer solved here and by Sliz et al., identifying the extended conformation. (b) Superimposition of the native EAA decamer, the P2-modified ELA decamer solved by Sliz et al., and the P1-modified LAG nonamer, identifying the bulged conformation. (c) Stereo image comparing the extended conformation of the native AAG nonamer and the bulged conformation of the ELA decamer. (d) Same as in (c), but rotated 90° out and showing the surface of HLA-A2 as partially transparent. All superimpositions are via the backbones of P1 and P6–P9.
Figure 2
Quantitative comparison of the conformations of the various MART-126/27–35-based peptides. The Figure shows the pair-wise superimposition matrix of all conformations of the peptides, including both molecules in each asymmetric unit for the structures solved here (MOL 1 and MOL 2), the two alternative conformations for the ALG nonamer (MOL 1A and MOL 1B), and the ALG and ELA structures of Sliz et al. Values are RMSD in Å. Superimpositions are via the backbones of P1–P9 (the first amino acid residue in the decameric peptides is P0). Values for peptides in the extended conformation (AAG and ALG) are green; values for peptides in the bulged conformation (EAA, ELA, and LAG) are blue. Cross-conformational superimpositions are red. Superimpositions of two molecules in the asymmetric units of any one structure (i.e. MOL 1 onto MOL 2) are shaded grey. It is of note that the cross-conformational superimpositions are all close to 2 Å, reflecting the differences between the bulged and extended conformations.
Figure 3
Conformational heterogeneity in the extended but not the bulged conformation. (a) Superimposition of all copies of the peptides in the extended conformation reveals conformational heterogeneity, particularly in the center of the peptide. (b) Close-up of the circled region in (a), rotated 180° around the vertical axis. The diversity in the position of the backbone at Gly31 is apparent, particularly the alternative conformation of the ALG nonamer (MOL 1B). (c) The conformational heterogeneity in the extended conformation can be accounted for mostly by considering the shift in the Gly31 α carbon atom. RMSD values from Figure 2 are plotted against the shift in the Gly31 α carbon atom relative to its position in ALG MOL 2 (green in (b)). (d) The conformational heterogeneity in the extended conformation does not indicate a propensity for the peptides to adopt the bulged conformation. All conformations of the ALG nonamer, which has the greatest conformational heterogeneity, are superimposed onto the conformation of the ELA decamer. (e) Superimposition of all copies of the peptides in the bulged conformation. All superimpositions are via the backbones of P1 and P6–P9.
Figure 4
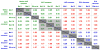
Molecular dynamic simulations indicate the ALG nonamer is mobile within the HLA-A2 peptide-binding groove. (a) _B_-factors averaged for each peptide backbone unit (N, CA, C, and O) computed from unrestrained, 30 ns molecular dynamic simulations of the AAG, ALG, and ELA peptide/HLA-A2 complexes indicates that the center of the ALG nonamer is highly mobile compared to the AAG and ELA peptides. (b) During the ALG nonamer simulation, the center of the peptide was found to populate a conformation similar to the alternative, “flipped” conformation observed crystallographically. This is demonstrated by plotting the Ψ angle of Ile30 (P4) versus the Φ angle of Gly31 (P5) for each step in the simulation and comparing the results with the crystallographically observed Φ/Ψ angles (indicated in the plot). The alternative, flipped conformation similar to that observed in ALG MOL 1B (circled in (b)) was observed for approximately 20% of simulation time. (c) In contrast to the ALG nonamer, the AAG nonamer did not adopt the alternative conformation, instead maintaining a conformation close to the single conformation observed crystallographically. For molecular dynamic simulations with the ALG and ELA peptide/HLA-A2 complexes, starting coordinates were from the structures described by Sliz et al. For the AAG simulations, starting coordinates were from the second molecule in the asymmetric unit (chains D, E, and F) from the structure reported here. Hydrogen atoms were added to the starting structures using the Protonate tool of the AMBER 8 suite [
]. Using the xLEaP tool, the structures were immersed in TIP3P water boxes such that no protein atoms were less than 12 Å from any side. Sodium cations were added for neutrality. This resulted in systems consisting of 19,567 atoms for the AAG system, 21,350 atoms for the ALG system, and 18,142 atoms for the ELA system. Dynamics simulations were then performed using the PMEMD module, with parameters from the parm99 set. Equilibration consisted of 10,000 steps of conjugate gradient energy minimization, followed by 20 ps of MD with restraints applied to the proteins to equilibrate the water. A series of energy minimizations was then carried out to relax the proteins, whereby the restraints were eliminated gradually. The systems were then warmed to 300 K over three MD simulations for a total of 480 ps of dynamics. This was followed by unrestrained production runs of 30 ns. The SHAKE algorithm was used, allowing a 2 fs time-step. Long-range electrostatics were treated via particle mesh Ewald. Trajectory analysis was carried out with the Ptraj tool and in-house scripts.
Figure 5
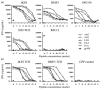
Cross-reactivity and specificity towards MART-126/27–35 variants with naturally occurring TIL and TCR RNA-electroporated PBL. (a) Four naturally occurring MART-127–35 reactive TIL (JKF6, DMF5, 1963-F6, 1992-W22) and the gp100209–217-specific T cell clone R6C12, were assayed for reactivity towards T2 APC pulsed with the MART-126/27–35 peptide variants or the gp100209–217 negative control peptide. JKF6 and DMF5 recognized all of the MART-126/27–35 variants studied, including the ALG nonamer. Similar to the findings reported by Valmori et al., 1963-F6 and 1922-W22 recognized the ALG nonamer either very poorly or not at all, despite the observation that the peptide adopts the same overall structure as the AAG nonamer. (b) Gene transfer of MART-1-specific TCR α and β chains from either the JKF6 and DMF5 T cells confers reactivity towards the MART-126/27–35 variants to otherwise non-reactive CD8+ PBL. TCR gene-transduced CD8+ T cells did not recognize the gp100209–217 negative control peptide. For cytokine secretion assays, cryopreserved TIL samples were thawed and cultured overnight in culture medium plus rhIL-2 (50 CU/ml). Responder cells were washed twice, plated at 1×105 cells, then co-cultured overnight with 1×105 HLA-A2+ T2 APC unpulsed or pulsed with titered concentrations of the AAG, ALG, LAG, EAA or ELA peptides or 1 μM gp100209–217 peptide. Co-culture supernatants were harvested and assessed for the presence of IFN-γ by ELISA assay in accordance with the manufacturer’s protocol (Pierce Endogen). Values reflect the mean of duplicate measurements and are reported in pg/ml. For TCR gene isolation, RNA was purified from T cell clones using Qiagen RNEasy. 5′RACE was performed using BD SmartRace, using the universal 5′ primer, and a 3′ gene-specific primer for the TCR α constant region, or C1 or C2 β constant regions. Products were separated by electrophoresis and appropriately sized bands were excised, subcloned into pCR2.1 (Invitrogen) vector, and sequenced. For in vitro TCR RNA transcription and expression in PBMC, gene-specific oligonucleotide primers were generated for the production of in vitro RNA transcription (IVT). The 5′ primer included the bacteriophage T7 polymerase binding sequence, followed immediately by a Kozak sequence, a start codon and the next 19–25 bp of Vα or Vβ region for each TCR gene; JKF6 TRVB28, DMF5 TRVB6.4. Vα regions were all 12.2 fwd 5′ TAATAC GAC TCA CTA TAG GGA GAA CCG CCA GCA AAT CCT TGA GAG GTT TAC 3′. Reverse primers included 64T and 18–25 bp of the relevant α or β constant region sequence. Reverse primers were Cα 5′ (64)T TTC AAC TGG ACC ACA GCC TCA GC 3′; C1-β 5′ (64)T TTC ATG AAT TCT TTC TTT TCA CC 3′ or C2-β 5′ (64)T TCT AGC CTC TGG AAT CCT TTC TCT TG 3′. For IVT, a PCR product was generated using the subcloned cDNA in pCR2.1 as template with the above oligonucleotide primer sets. Resulting bands were gel-purified and used for a second round of PCR amplification. PCR product was cleaned using Zymogen DNA purification columns. A 1–3 μg sample of PCR product was used as template for IVT using Ambion T7 mMESSAGE MACHINE according to the manufacturer’s instructions, followed by RNA cleanup using Qiagen RNEasy. In preparation for RNA electroporation, donor TIL or PBMC from phereses were stimulated in vitro with 50 ng/ml OKT-3, 50 CU IL-2 in STEM:RPMI medium for three days, when CD8+ cells were positively selected using Miltenyi Biotech microbeads and magnetic columns. PBMC were then grown in vitro an additional 2–15 days in IL-2-containing medium before use. For TCR electroporation, 2.0 μg of RNA from each TCR gene was used per 1×106 cells at 2.5×107 cells/ml in Opti-MEM serum-free medium (Invitrogen). Cells were rested for 2 h without IL-2 post-electroporation before use in FACS staining or co-culture experiments.
Similar articles
- Crystal structures of HLA-A*0201 complexed with Melan-A/MART-1(26(27L)-35) peptidomimetics reveal conformational heterogeneity and highlight degeneracy of T cell recognition.
Douat-Casassus C, Borbulevych O, Tarbe M, Gervois N, Jotereau F, Baker BM, Quideau S. Douat-Casassus C, et al. J Med Chem. 2010 Oct 14;53(19):7061-6. doi: 10.1021/jm100683p. J Med Chem. 2010. PMID: 20806940 Free PMC article. - Crystal structures of two closely related but antigenically distinct HLA-A2/melanocyte-melanoma tumor-antigen peptide complexes.
Sliz P, Michielin O, Cerottini JC, Luescher I, Romero P, Karplus M, Wiley DC. Sliz P, et al. J Immunol. 2001 Sep 15;167(6):3276-84. doi: 10.4049/jimmunol.167.6.3276. J Immunol. 2001. PMID: 11544315 - In silico and cell-based analyses reveal strong divergence between prediction and observation of T-cell-recognized tumor antigen T-cell epitopes.
Schmidt J, Guillaume P, Dojcinovic D, Karbach J, Coukos G, Luescher I. Schmidt J, et al. J Biol Chem. 2017 Jul 14;292(28):11840-11849. doi: 10.1074/jbc.M117.789511. Epub 2017 May 23. J Biol Chem. 2017. PMID: 28536262 Free PMC article. - The structural interactions between T cell receptors and MHC-peptide complexes place physical limits on self-nonself discrimination.
Wucherpfennig KW. Wucherpfennig KW. Curr Top Microbiol Immunol. 2005;296:19-37. doi: 10.1007/3-540-30791-5_2. Curr Top Microbiol Immunol. 2005. PMID: 16329190 Review. - Structural and dynamic control of T-cell receptor specificity, cross-reactivity, and binding mechanism.
Baker BM, Scott DR, Blevins SJ, Hawse WF. Baker BM, et al. Immunol Rev. 2012 Nov;250(1):10-31. doi: 10.1111/j.1600-065X.2012.01165.x. Immunol Rev. 2012. PMID: 23046120 Review.
Cited by
- Differential modulation of mutant CALR and JAK2 V617F-driven oncogenesis by HLA genotype in myeloproliferative neoplasms.
Shivarov V, Tsvetkova G, Micheva I, Hadjiev E, Petrova J, Ivanova A, Madjarova G, Ivanova M. Shivarov V, et al. Front Immunol. 2024 Sep 16;15:1427810. doi: 10.3389/fimmu.2024.1427810. eCollection 2024. Front Immunol. 2024. PMID: 39351227 Free PMC article. - Dynamic allostery in the peptide/MHC complex enables TCR neoantigen selectivity.
Ma J, Ayres CM, Brambley CA, Chandran SS, Rosales TJ, Corcelli SA, Kovrigin EL, Klebanoff CA, Baker BM. Ma J, et al. Res Sq [Preprint]. 2024 May 29:rs.3.rs-4457195. doi: 10.21203/rs.3.rs-4457195/v1. Res Sq. 2024. PMID: 38854019 Free PMC article. Preprint. - Transfer learning improves pMHC kinetic stability and immunogenicity predictions.
Fasoulis R, Rigo MM, Antunes DA, Paliouras G, Kavraki LE. Fasoulis R, et al. Immunoinformatics (Amst). 2024 Mar;13:100030. doi: 10.1016/j.immuno.2023.100030. Epub 2023 Dec 21. Immunoinformatics (Amst). 2024. PMID: 38577265 Free PMC article. - Unconventional modes of peptide-HLA-I presentation change the rules of TCR engagement.
Hopkins JR, MacLachlan BJ, Harper S, Sewell AK, Cole DK. Hopkins JR, et al. Discov Immunol. 2022 May 4;1(1):kyac001. doi: 10.1093/discim/kyac001. eCollection 2022. Discov Immunol. 2022. PMID: 38566908 Free PMC article. Review. - APE-Gen2.0: Expanding Rapid Class I Peptide-Major Histocompatibility Complex Modeling to Post-Translational Modifications and Noncanonical Peptide Geometries.
Fasoulis R, Rigo MM, Lizée G, Antunes DA, Kavraki LE. Fasoulis R, et al. J Chem Inf Model. 2024 Mar 11;64(5):1730-1750. doi: 10.1021/acs.jcim.3c01667. Epub 2024 Feb 28. J Chem Inf Model. 2024. PMID: 38415656
References
- Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. - PubMed
- Kersh GJ, Miley MJ, Nelson CA, Grakoui A, Horvath S, Donermeyer DL, et al. Structural and functional consequences of altering a peptide MHC anchor residue. J Immunol. 2001;166:3345–3354. - PubMed
- Sharma AK, Kuhns JJ, Yan S, Friedline RH, Long B, Tisch R, Collins EJ. Class I major histocompatibility complex anchor substitutions alter the conformation of T cell receptor contacts. J Biol Chem. 2001;276:21443–21449. - PubMed
- Kirksey TJ, Pogue-Caley RR, Frelinger JA, Collins EJ. The structural basis for the increased immunogenicity of two HIV-reverse transcriptase peptide variant/class i major histocompatibility complexes. J Biol Chem. 1999;274:37259–37264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 BC010763-01/Intramural NIH HHS/United States
- Z99 CA999999/Intramural NIH HHS/United States
- Y1-CO-1020/CO/NCI NIH HHS/United States
- Y1-GM-1104/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials