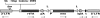Large-scale, saturating insertional mutagenesis of the mouse genome - PubMed (original) (raw)
. 2007 Sep 4;104(36):14406-11.
doi: 10.1073/pnas.0700608104. Epub 2007 Aug 24.
Kyoji Horie, Maria Pavlova, Linda Madisen, Hongkui Zeng, Galina Gragerova, Alex Rhode, Io Dolka, Patricia Roth, Amanda Ebbert, Stephanie Moe, Christopher Navas, Eric Finn, John Bergmann, Demetrios K Vassilatis, George N Pavlakis, George A Gaitanaris
Affiliations
- PMID: 17720809
- PMCID: PMC1964832
- DOI: 10.1073/pnas.0700608104
Large-scale, saturating insertional mutagenesis of the mouse genome
Alexander Gragerov et al. Proc Natl Acad Sci U S A. 2007.
Abstract
We describe the construction of a large-scale, orderly assembly of mutant ES cells, generated with retroviral insertions and having mutational coverage in >90% of mouse genes. We also describe a method for isolating ES cell clones with mutations in specific genes of interest from this library. This approach, which combines saturating random mutagenesis with targeted selection of mutations in the genes of interest, was successfully applied to the gene families of G protein-coupled receptors (GPCRs) and nuclear receptors. Mutant mouse strains in 60 different GPCRs were generated. Applicability of the technique for the GPCR genes, which on average represent fairly small targets for insertional mutagenesis, indicates the general utility of our approach for the rest of the genome. The method also allows for increased scale and automation for the large-scale production of mutant mice, which could substantially expedite the functional characterization of the mouse genome.
Conflict of interest statement
Conflict of interest statement: A.G., M.P., L.M., H.Z., G.G., A.R., I.D., A.E., S.M., C.N., E.F., J.B., D.K.V., and G.A.G. are or were employees of privately owned Omeros Corporation and declare competing financial interests.
Figures
Fig. 1.
Vector for insertional mutagenesis. The vector consists of the following components i–xi. (i) Packaging and integration sequences based on the Moloney murine leukemia virus. The vector lacks the viral enhancers and contains the bacterial supF gene in the 3′ LTR. Upon genome integration, the 5′ LTR enhancer is also deleted (Δen), and the supF sequence is copied to the 5′ LTR. (ii) The adenovirus major late transcript splice acceptor (SA) is included to facilitate the fusion of retroviral transcripts to the endogenous gene transcript in situations where retroviral integration occurs within an intron. (iii) Nonsense codons in all three reading frames ensure translational termination. (iv) The internal ribosome entry site (IRES) from the encephalomyocarditis virus provides translation initiation of the rtTA gene. (v) The reverse tetracycline transactivator (rtTA) stimulates the expression of genes placed under the control of the tetracycline operator in the presence of tetracycline derivatives (27). rtTA is expressed under the control of the endogenous gene, which has been mutated by the insertion. Although not essential for KO generation, rtTA is a key component of an inducible KO system (H.Z., K.H., L.M., M.P., G.G., A.R., B. Shimpf, Y. Liang, E. Ojala, F. Kramer, P.R., O. Slobodskaya, I.D., E. Southon, L. Tessarollo, K. Bornfeldt, A.G., G.N.P., and G.A.G., unpublished work). (vi) Polyadenylation signal (pA) from the bovine growth hormone gene provides for the expression of rtTA mRNA. (vii) The recognition sequences of bacteriophage P1 Cre recombinase LoxP (L) provide the option of removing the phosphoglycerate kinase (PGK) promoter (P) and neomycin phosphotransferase (neo), if desired. (viii) The PGK promoter (P) drives the expression of neo. (ix) The neo-selectable marker renders ES cells containing the provirus resistant to G418. (x) Synthetic poly(A) signal (spA) facilitates the expression of the neo mRNA. (xi) Also included is the transcription terminator (t) from the human complement gene (28) to terminate transcription from both the PGK and cellular promoters.
Fig. 2.
Pooling scheme for a single library unit. Equal amounts of cell suspension from wells of a group of 10 96-well plates were transferred to the following: (i) deep-well tube racks with 2× freezing medium for storage and later retrieval (data not shown), (ii) 15-cm dish combining each well of each 96-well plate to make plate pools P1–P10, (iii) 15-cm dish combining each well from a given column from all of the plates in the group to make column pools C1–C12, and (iv) 15-cm dish combining each well from a given row from all of the plates in the group to make row pools RA–RH. Deep-well plates were stored in liquid nitrogen. Pooled cells were grown and treated in the following way. Half of each column and row pool was used to prepare three freezing vials for the future regrowth of the pools. The other half was used for genomic DNA isolation. Each plate pool was split at a ratio of 1:3 to 3 × 15-cm dishes (total of 30 dishes per library unit) and, after growth, each was processed the same way as the column and row pools. This step was needed to produce more DNA and more frozen copies of the pools for later regrowth, because plate pool DNAs were used the most during library screening for the inactivation of specific genes.
Fig. 3.
Library screening. (A) Nested PCR with gene- and vector-specific primers (Top) was used to screen plate pools from multiple library units (Middle Left). One lane corresponds to one plate pool. The example shows screening of four units, with 10 plate pools in each unit for a gene of interest. Multiple insertions (PCR bands) were detected in different plate pools. Then column and row pools (Middle Center and Right, respectively) of the unit(s) that demonstrated the presence of a sequencing-confirmed insertion in the gene were screened by using the same pairs of primers to find the 3D address of the positive well in the library (e.g., the positive well shown at Bottom gave rise to identical PCR fragments in plate pool no. 22, column pool no. 9, row pool A). (B) The results of screening eight library units (≈4 × 106 mutant ES cell clones) for insertions in the genes of two different GPCRs. M, marker.
Fig. 4.
Inactivation of the HPRT gene by retroviral insertions. (A) Positions of vector insertions in HPRT, an X-linked gene, which allowed the direct assessment of gene inactivation in ES cells. Insertion locations were determined by sequencing vector–genome junctions for individual insertions. (B) HPRT activity of 10 ES cell clones marked by numbers in A. Activity was determined by the ability of clones to grow in the presence of 6-thioguanine (6-TG). All but one of the clones, no. 7, showed a lack of HPRT activity.
Fig. 5.
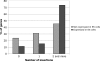
Ability of the retroviral vector to inactivate genes that are expressed in ES cells as well as silent genes. Frequencies of insertions into 53 GPCR genes expressed in ES cells (active genes) and 55 GPCR genes not expressed in ES cells (inactive genes) are compared and demonstrate similar potentials for these loci to be targets for the vector integration. Overall, 89% of active genes and 77% of inactive genes had one or more viral insertions.
Similar articles
- Selection of targeted mutants from a library of randomly mutagenized ES cells.
Horie K, Gaitanaris G, Gragerov A. Horie K, et al. Methods Mol Biol. 2011;693:283-94. doi: 10.1007/978-1-60761-974-1_17. Methods Mol Biol. 2011. PMID: 21080287 - Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells.
Zambrowicz BP, Friedrich GA, Buxton EC, Lilleberg SL, Person C, Sands AT. Zambrowicz BP, et al. Nature. 1998 Apr 9;392(6676):608-11. doi: 10.1038/33423. Nature. 1998. PMID: 9560157 - Insertional versus targeted mutagenesis in mice.
Gridley T. Gridley T. New Biol. 1991 Nov;3(11):1025-34. New Biol. 1991. PMID: 1777476 Review. - Gene targeting in murine embryonic stem cells: introduction of specific alterations into the mammalian genome.
Mansour SL. Mansour SL. Genet Anal Tech Appl. 1990 Dec;7(8):219-27. doi: 10.1016/0735-0651(90)90004-y. Genet Anal Tech Appl. 1990. PMID: 2091698 Review.
Cited by
- Kiss of the mutant mouse: how genetically altered mice advanced our understanding of kisspeptin's role in reproductive physiology.
Dungan Lemko HM, Elias CF. Dungan Lemko HM, et al. Endocrinology. 2012 Nov;153(11):5119-29. doi: 10.1210/en.2012-1494. Epub 2012 Sep 25. Endocrinology. 2012. PMID: 23011921 Free PMC article. Review. - An inducible and reversible mouse genetic rescue system.
Zeng H, Horie K, Madisen L, Pavlova MN, Gragerova G, Rohde AD, Schimpf BA, Liang Y, Ojala E, Kramer F, Roth P, Slobodskaya O, Dolka I, Southon EA, Tessarollo L, Bornfeldt KE, Gragerov A, Pavlakis GN, Gaitanaris GA. Zeng H, et al. PLoS Genet. 2008 May 9;4(5):e1000069. doi: 10.1371/journal.pgen.1000069. PLoS Genet. 2008. PMID: 18464897 Free PMC article. - A simplified transposon mutagenesis method to perform phenotypic forward genetic screens in cultured cells.
Feddersen CR, Wadsworth LS, Zhu EY, Vaughn HR, Voigt AP, Riordan JD, Dupuy AJ. Feddersen CR, et al. BMC Genomics. 2019 Jun 17;20(1):497. doi: 10.1186/s12864-019-5888-6. BMC Genomics. 2019. PMID: 31208320 Free PMC article. - Large-scale gene trapping in C57BL/6N mouse embryonic stem cells.
Hansen GM, Markesich DC, Burnett MB, Zhu Q, Dionne KM, Richter LJ, Finnell RH, Sands AT, Zambrowicz BP, Abuin A. Hansen GM, et al. Genome Res. 2008 Oct;18(10):1670-9. doi: 10.1101/gr.078352.108. Epub 2008 Sep 17. Genome Res. 2008. PMID: 18799693 Free PMC article. - Gene trap mutagenesis in the mouse.
Friedel RH, Soriano P. Friedel RH, et al. Methods Enzymol. 2010;477:243-69. doi: 10.1016/S0076-6879(10)77013-0. Methods Enzymol. 2010. PMID: 20699145 Free PMC article.
References
- Thomas KR, Folger KR, Capecchi MR. Cell. 1986;44:419–428. - PubMed
- Copeland NG, Jenkins NA, Court DL. Nat Rev Genet. 2001;2:769–779. - PubMed
- Heintz N. Nat Rev Neurosci. 2001;2:861–870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases