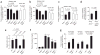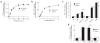Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype - PubMed (original) (raw)
. 2007 Sep;13(9):1086-95.
doi: 10.1038/nm1626. Epub 2007 Aug 26.
Tatiana V Byzova, Maria Febbraio, Robert G Salomon, Yi Ma, Manojkumar Valiyaveettil, Eugenia Poliakov, Mingjiang Sun, Paula J Finton, Brian R Curtis, Juhua Chen, Renliang Zhang, Roy L Silverstein, Stanley L Hazen
Affiliations
- PMID: 17721545
- PMCID: PMC3042888
- DOI: 10.1038/nm1626
Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype
Eugene A Podrez et al. Nat Med. 2007 Sep.
Abstract
Dyslipidemia is associated with a prothrombotic phenotype; however, the mechanisms responsible for enhanced platelet reactivity remain unclear. Proatherosclerotic lipid abnormalities are associated with both enhanced oxidant stress and the generation of biologically active oxidized lipids, including potential ligands for the scavenger receptor CD36, a major platelet glycoprotein. Using multiple mouse in vivo thrombosis models, we now demonstrate that genetic deletion of Cd36 protects mice from hyperlipidemia-associated enhanced platelet reactivity and the accompanying prothrombotic phenotype. Structurally defined oxidized choline glycerophospholipids that serve as high-affinity ligands for CD36 were at markedly increased levels in the plasma of hyperlipidemic mice and in the plasma of humans with low HDL levels, were able to bind platelets via CD36 and, at pathophysiological levels, promoted platelet activation via CD36. Thus, interactions of platelet CD36 with specific endogenous oxidized lipids play a crucial role in the well-known clinical associations between dyslipidemia, oxidant stress and a prothrombotic phenotype.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Figures
Figure 1
CD36 plays a role in thrombosis in vivo in the setting of hypercholesterolemia. (a–c) Mice of the indicated genotypes were maintained on Western diet (solid bars) and then used for an intravital thrombosis assay. Mesenteric arterioles (a) or venules (b) or carotid arteries (c) were visualized, and in vivo thrombosis times were measured as described in Methods. Mice on chow diet (empty bars) were analyzed in the same way, except that FeCl3 exposure times were increased by 1 min. Data are presented as mean ± s.e.m. for n ≥ 7 for chow diet and n ≥ 8 for Western diet. (d) _Apoe_−/− mice and _Apoe_−/− _Cd36_−/− on a Western diet were depleted of platelets by γ-irradiation, subsequently injected with platelets from either _Apoe_−/− mice (n = 5) or _Apoe_−/− _Cd36_−/− mice (n = 5) and the carotid artery occlusion test was performed as described in Methods. (e) Tail vein bleeding assay was performed as described in Methods. Data are presented as mean ± s.e.m.; numbers of mice used are: wild-type (n = 8), _Cd36_−/− (n = 5), _Apoe_−/− (n = 4) and _Cd36_−/− _Apoe_−/− (n = 4). (f) Total plasma cholesterol concentration is presented as mean ± s.d. mg/dl for at least 6 animals with the indicated genotype on a Western diet. ***P < 0.001 by _t_-test as compared to wild-type. (g) Indicated groups of mice were fed a high-cholesterol diet as described in Methods, and in vivo thrombosis times were assessed as in a–c. n ≥ 7 animals in each group. See Supplementary Methods for details.
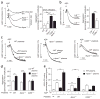
Figure 2
CD36 deficiency blunts platelet responses to agonists in hypercholesterolemic plasma. (a,b) Platelet aggregation in platelet-rich plasma from mice of the indicated genotypes on the indicated diets was induced by ADP and optically monitored. Representative aggregation curves are shown. Bar graph shows aggregation results expressed as maximal amplitude of aggregation (mean ± s.e.m.; n = 3 for WT, n = 4 for _Apoe_−/−, n = 6 for _Apoe_−/− _Cd36_−/−, n = 3 for Ldlr−/− and n = 4 for _Ldlr_−/− Cd36−/−). (c) Platelets were isolated by gel filtration of pooled blood from wild-type and _Cd36_−/− mice on a chow diet and _Apoe_−/− mice on a Western diet as described in Methods. Citrated, pooled, platelet-poor plasma from either wild-type or _Apoe_−/− mice on a Western diet was isolated and combined with platelets of the indicated genotype at a 1:1 ratio. Aggregation was induced by ADP and optically monitored. Representative aggregation curves are shown. (d) Bar graph shows aggregation results from c expressed as maximal amplitude of aggregation (mean ± s.e.m.; n = 3 for WT, n = 3 for _Apoe_−/− and n = 4 for _Cd36_−/−). (e) Flow cytometric analysis of integrin αIIbβ3 activation on mouse platelets. Platelets and citrated, platelet-poor plasma were isolated and combined at a 1:2 ratio (vol/vol), stimulated with ADP and integrin αIIbβ3 activation was assessed using JON/A, a monoclonal, phycoerythrin-conjugated antibody for mouse αIIbβ3 in the activated conformation (mean ± s.e.m.; n = 3 for WT, n = 3 for _Apoe_−/−, n = 4 for _Cd36_−/−). *P < 0.05, **P < 0.01 and ***P < 0.001. See Supplementary Methods for details.
Figure 3
Platelet CD36 specifically binds oxPCCD36 and LDL oxidized by MPO−H2O2−NO2− system. (a) [125I]LDL modified by the indicated methods was incubated with platelets isolated from humans in Tyrode’s buffer in the presence of 2 mM Ca2+ at room temperature. Unbound [125I]LDL was separated from platelets and the bound radioactivity was quantified. NO2-LDL, LDL oxidized by the MPO-H2O2-NO2− system; AcetLDL, acetylated LDL; Cu-oxLDL, Cu2+-oxidized LDL. (b) Concentration dependence of [125I]NO2-LDL binding by platelets. Binding of [125I]LDL was used as a control. (c) Human platelets were fixed with paraformaldehyde and incubated with [125I]NO2-LDL in the presence or absence of the indicated antibodies (20 μg/ml), and the bound radioactivity was assessed. (d) Binding of native [125I]LDL and [125I]NO2-LDL by isolated platelets from _Cd36_−/− and wild-type mice was assessed as in a. (e) Binding of [125I]NO2-LDL to human platelets in the presence of various concentrations of the indicated phospholipids was determined as in a. (f) Direct binding of vesicles containing 40 mol % of PAPC (for KOOA-PC) or 40 mol % of PLPC (for KODA-PC), 40 mol % of the indicated specific native phospholipid or oxPCCD36, 20 mol % of cholesterol, and trace amounts of [3H]DPPC (1,2-dihexadecanoyl-_sn_-glycero-3-phosphocholine) were generated and then incubated with platelets alone or, in the case of KODA-PC–containing vesicles, in the presence of either CD36-specific monoclonal antibody (FA6) or nonimmune, isotype-matched antibody (NI IgG). Unbound vesicles were separated and platelet-bound radioactivity was then quantified. Results represent the mean ± s.d. of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001. See Supplementary Methods for details.
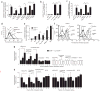
Figure 4
oxPCCD36 activates platelet fibrinogen receptor integrin αIIbβ3 in a CD36-dependent manner. (a) Human platelets isolated by gel filtration were incubated with increasing concentrations of oxPCCD36 or OV-PC and αIIbβ3 activation was assessed on the basis of the binding of 125I-labeled fibrinogen. (b) Human platelets isolated by gel filtration were incubated with increasing concentrations of oxPCCD36 or OV-PC. After addition of agonist, FITC-labeled PAC-1 mouse monoclonal antibody (specific for activated integrin αIIbβ3) was added at a dilution of 1:100 and αIIbβ3 activation was assessed by FACS analysis. (c) Human platelets were isolated from CD36+/+ or _CD36_−/− donors and analyzed for αIIbβ3 activation as in b. The final concentrations of stimuli used were 10 μM ADP, 20 μM lipid oxidized phospholipids, 0.5 U/ml thrombin and 10 nM PMA. NA, no additions. (d) Washed platelets from wild-type or _Cd36_−/− mice were incubated with 10 μM ADP or 20 μM synthetic oxidized phospholipids, and platelet activation was assessed by 125I-labeled fibrinogen binding. NA, no additions. Results represent the mean ± s.d. of three independent experiments. Statistical significance was determined by _t_-test: *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Methods for details.
Figure 5
oxPCCD36 induce platelet P-selectin expression in a CD36-dependent manner. (a) Isolated human platelets were incubated with the indicated stimuli and P-selectin expression was assessed as described in Methods. NA, no additions. Results represent the mean ± s.d. of three independent experiments. 5–144 aa, CD36-GST fusion peptide with amino acids 5–144 of CD36; GST, glutathione _S_-transferase. (b) Binding of 3H-labeled small unilamellar vesicles containing oxPCCD36 (HODA-PC) to recombinant CD36-GST fusion peptides immobilized on glutathione-Sepharose was assessed in the presence or absence of the indicated competitors. 93–120 aa, CD36-GST fusion peptide with amino acids 93–120 of CD36. (c,d) Effect of the Fab fragment of the CD36-blocking monoclonal antibody on platelet activation induced by (c) NO2-LDL and NO2-PAPC and (d) oxPCCD36. NI, nonimmune isotype-matched antibody. (e) Platelet P-selectin expression was determined using FITC-conjugated antibody to mouse P-selectin. (f) Human platelets isolated from CD36+/+ or _CD36_−/− donors were treated with agonists and P-selectin expression was assessed. (b,c,e) Data are presented as mean ± s.d. of three independent experiments; (d,f) data are presented as a typical result of at least three independent experiments. (g) Human platelets from CD36+/+ or _CD36_−/− donors (n = 3) were combined with human citrated plasma from various donors, stimulated with ADP, and P-selectin expression was assessed. Samples were divided into tertiles according to P-selectin expression, the mean plasma sample lipid levels were assigned a relative value of 1 for the lowest P-selectin tertile, and the means ± s.e.m. are shown for all P-selectin tertiles. (h) Human plasma samples (n = 24) were analyzed for levels of multiple oxidized phospholipids, HDL cholesterol and LDL cholesterol. Samples were divided into tertiles according to HDL cholesterol or LDL cholesterol concentrations. The mean oxidized phospholipid levels were assigned relative values of 1 for the lowest lipoprotein tertile, and corresponding mean ± s.e.m. are shown for all lipoprotein tertiles. *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Methods for details.
Comment in
- The clot thickens--oxidized lipids and thrombosis.
Jackson SP, Calkin AC. Jackson SP, et al. Nat Med. 2007 Sep;13(9):1015-6. doi: 10.1038/nm0907-1015. Nat Med. 2007. PMID: 17828215 No abstract available.
Similar articles
- TLR2 Plays a Key Role in Platelet Hyperreactivity and Accelerated Thrombosis Associated With Hyperlipidemia.
Biswas S, Zimman A, Gao D, Byzova TV, Podrez EA. Biswas S, et al. Circ Res. 2017 Sep 29;121(8):951-962. doi: 10.1161/CIRCRESAHA.117.311069. Epub 2017 Aug 3. Circ Res. 2017. PMID: 28775078 Free PMC article. - Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions.
Yang M, Cooley BC, Li W, Chen Y, Vasquez-Vivar J, Scoggins NO, Cameron SJ, Morrell CN, Silverstein RL. Yang M, et al. Blood. 2017 May 25;129(21):2917-2927. doi: 10.1182/blood-2016-11-750133. Epub 2017 Mar 23. Blood. 2017. PMID: 28336528 Free PMC article. - Regulation of platelet function by class B scavenger receptors in hyperlipidemia.
Zimman A, Podrez EA. Zimman A, et al. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2350-6. doi: 10.1161/ATVBAHA.110.207498. Epub 2010 Nov 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 21071700 Free PMC article. Review. - Platelet CD36 signaling through ERK5 promotes caspase-dependent procoagulant activity and fibrin deposition in vivo.
Yang M, Kholmukhamedov A, Schulte ML, Cooley BC, Scoggins NO, Wood JP, Cameron SJ, Morrell CN, Jobe SM, Silverstein RL. Yang M, et al. Blood Adv. 2018 Nov 13;2(21):2848-2861. doi: 10.1182/bloodadvances.2018025411. Blood Adv. 2018. PMID: 30381401 Free PMC article. - HDL scavenger receptor class B type I and platelet function.
Nofer JR, van Eck M. Nofer JR, et al. Curr Opin Lipidol. 2011 Aug;22(4):277-82. doi: 10.1097/MOL.0b013e32834701de. Curr Opin Lipidol. 2011. PMID: 21537173 Review.
Cited by
- Platelets and Their Role in the Pathogenesis of Cardiovascular Events in Patients With Community-Acquired Pneumonia.
Feldman C, Anderson R. Feldman C, et al. Front Immunol. 2020 Sep 17;11:577303. doi: 10.3389/fimmu.2020.577303. eCollection 2020. Front Immunol. 2020. PMID: 33042161 Free PMC article. Review. - Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling.
Zhou MS, Chadipiralla K, Mendez AJ, Jaimes EA, Silverstein RL, Webster K, Raij L. Zhou MS, et al. Am J Physiol Heart Circ Physiol. 2013 Aug 15;305(4):H563-74. doi: 10.1152/ajpheart.00042.2013. Epub 2013 Jun 7. Am J Physiol Heart Circ Physiol. 2013. PMID: 23748423 Free PMC article. - Sources of tissue factor that contribute to thrombosis after rupture of an atherosclerotic plaque.
Owens AP 3rd, Mackman N. Owens AP 3rd, et al. Thromb Res. 2012 May;129 Suppl 2(Suppl 2):S30-3. doi: 10.1016/j.thromres.2012.02.026. Epub 2012 Mar 22. Thromb Res. 2012. PMID: 22444158 Free PMC article. Review. - Scavenger receptors in homeostasis and immunity.
Canton J, Neculai D, Grinstein S. Canton J, et al. Nat Rev Immunol. 2013 Sep;13(9):621-34. doi: 10.1038/nri3515. Epub 2013 Aug 9. Nat Rev Immunol. 2013. PMID: 23928573 Review. - Oxidised Low-Density Lipoprotein-Induced Platelet Hyperactivity-Receptors and Signalling Mechanisms.
Berger M, Naseem KM. Berger M, et al. Int J Mol Sci. 2022 Aug 16;23(16):9199. doi: 10.3390/ijms23169199. Int J Mol Sci. 2022. PMID: 36012465 Free PMC article. Review.
References
- Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. - PubMed
- Lacoste L, et al. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. - PubMed
- Kabbani SS, et al. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001;104:181–186. - PubMed
- Vanschoonbeek K, et al. Thrombin-induced hyperactivity of platelets of young stroke patients: involvement of thrombin receptors in the subject-dependent variability in Ca2+ signal generation. Thromb Haemost. 2002;88:931–937. - PubMed
- Kabbani SS, et al. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. Am J Cardiol. 2003;91:876–878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL077107/HL/NHLBI NIH HHS/United States
- P50 HL81011/HL/NHLBI NIH HHS/United States
- P01 HL046403/HL/NHLBI NIH HHS/United States
- R01 HL071625/HL/NHLBI NIH HHS/United States
- R01 HL071625-05/HL/NHLBI NIH HHS/United States
- HL053315/HL/NHLBI NIH HHS/United States
- HL46403/HL/NHLBI NIH HHS/United States
- HL072942/HL/NHLBI NIH HHS/United States
- R01 HL070621/HL/NHLBI NIH HHS/United States
- P01 HL072942/HL/NHLBI NIH HHS/United States
- R01 HL053315/HL/NHLBI NIH HHS/United States
- HL076491/HL/NHLBI NIH HHS/United States
- HL70621/HL/NHLBI NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- R01 HL077213-05/HL/NHLBI NIH HHS/United States
- P01 HL073311-050004/HL/NHLBI NIH HHS/United States
- HL077213/HL/NHLBI NIH HHS/United States
- R01 HL070083/HL/NHLBI NIH HHS/United States
- P01 HL073311/HL/NHLBI NIH HHS/United States
- P50 HL081011/HL/NHLBI NIH HHS/United States
- HL071625/HL/NHLBI NIH HHS/United States
- M01 RR018390/RR/NCRR NIH HHS/United States
- R01 HL077213/HL/NHLBI NIH HHS/United States
- HL073311/HL/NHLBI NIH HHS/United States
- HL 70083/HL/NHLBI NIH HHS/United States
- P50 HL077107/HL/NHLBI NIH HHS/United States
- HL077107/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases