Germinal-center organization and cellular dynamics - PubMed (original) (raw)
Review
Germinal-center organization and cellular dynamics
Christopher D C Allen et al. Immunity. 2007 Aug.
Abstract
Germinal centers (GCs) are important sites of antibody affinity maturation. In the classical model, the GC dark zone contains large centroblasts that are rapidly proliferating and undergoing somatic hypermutation of their antibody variable-region genes. Centroblasts give rise to smaller nonproliferating centrocytes in the light zone that compete for binding antigen on follicular dendritic cells. Recently, the approach of real-time imaging of GCs by two-photon microscopy of intact lymph nodes has provided new insights into GC dynamics that both support and challenge fundamental aspects of this model. Here we review recent and older findings on cell migration, proliferation, and interaction dynamics in the GC and discuss a model in which dark- and light-zone cells are morphologically similar, proliferation occurs in both zones, and GC B cells compete for T cell help as well as antigen.
Figures
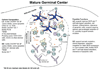
Figure 1.. Schematic representation of GC compartments and cellular dynamics
The cellular composition of the compartments is summarized on the left and possible functions of the compartments are described on the right. The diagram depicts the structure of an acute GC but chronic GCs, such as those typical of tonsils, may have additional levels of compartmentalization. GC B cells are medium sized blasts expressing low surface Ig and exhibiting a dendritic morphology, in both the dark and light zones. In humans, dark zone cells also express somewhat higher amounts of CD77 (Hardie et al., 1993) whereas CD44 may be higher on light zone cells (Feuillard et al., 1995). Additional cell types may sometimes be present including dendritic cells (Grouard et al., 1996), and CD4+CD3− cells (Kim et al., 2003). GC B cells migrate extensively within their respective compartments and move between compartments, most likely after modulating CXCR4 protein levels. Blebs of apoptotic GC B cells can associate with T cells, possibly limiting the availability of T cell help. Antigen is displayed as immune complexes (ICs) on FDCs but also possibly on tingible body macrophages (TBMØs) and motile B cells. Soluble antigen may also be present. Note the greater cellular heterogeneity and thus lower GC B cell density in the light zone than in the dark zone. See text for further details.
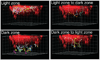
Figure 2.. GC B cell movement within and between GC dark and light zones
The light zone FDCs are identified by in vivo deposited PE immune complexes (red). Colored lines show tracks of GC B cells that moved within the light or dark zones or that crossed between zones, as indicated, during a 30 minute imaging session. The gridlines are separated by 20µm. Cell tracks were manually classified as being in the light or dark zones if the entire track stayed within the PE+ or PE− region, respectively, during the imaging session. Cell tracks were manually classified as traveling between zones if the tracks originated in one zone, crossed an approximately 20 µm border between the zones, then entered at least 10 µm into the other zone, and stayed in that zone for the duration of the analysis. Some tracks could not be definitively classified by these criteria, for example tracks that were too close to the border between the light and dark zones. From Allen et al., Science 315: 528 (2007).
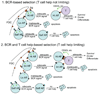
Figure 3.. Two models of GC B cell selection within the GC
In the first model, discrimination between cells of different affinity for the foreign antigen occurs solely at the level of different strengths of BCR signaling. In the second model, sufficient BCR engagement is still necessary but further selection occurs due to competition for T cell help. B cells that have captured, processed, and presented more antigen as MHC-peptide complexes go on to receive T cell help at the expense of cells that have captured less antigen. See text for details.
Similar articles
- Accumulation of somatic hypermutation and antigen-driven selection in rapidly cycling surface Ig+ germinal center (GC) B cells which occupy GC at a high frequency during the primary anti-hapten response in mice.
Kimoto H, Nagaoka H, Adachi Y, Mizuochi T, Azuma T, Yagi T, Sata T, Yonehara S, Tsunetsugu-Yokota Y, Taniguchi M, Takemori T. Kimoto H, et al. Eur J Immunol. 1997 Jan;27(1):268-79. doi: 10.1002/eji.1830270140. Eur J Immunol. 1997. PMID: 9022029 - In vivo imaging of germinal centres reveals a dynamic open structure.
Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. Schwickert TA, et al. Nature. 2007 Mar 1;446(7131):83-7. doi: 10.1038/nature05573. Epub 2007 Jan 31. Nature. 2007. PMID: 17268470 - Imaging of germinal center selection events during affinity maturation.
Allen CD, Okada T, Tang HL, Cyster JG. Allen CD, et al. Science. 2007 Jan 26;315(5811):528-31. doi: 10.1126/science.1136736. Epub 2006 Dec 21. Science. 2007. PMID: 17185562 - Maturation and dispersal of B-cell clones during T cell-dependent antibody responses.
MacLennan IC, Liu YJ, Johnson GD. MacLennan IC, et al. Immunol Rev. 1992 Apr;126:143-61. doi: 10.1111/j.1600-065x.1992.tb00635.x. Immunol Rev. 1992. PMID: 1597318 Review. - System-Level Scenarios for the Elucidation of T Cell-Mediated Germinal Center B Cell Differentiation.
Verstegen NJM, Ubels V, Westerhoff HV, van Ham SM, Barberis M. Verstegen NJM, et al. Front Immunol. 2021 Sep 20;12:734282. doi: 10.3389/fimmu.2021.734282. eCollection 2021. Front Immunol. 2021. PMID: 34616402 Free PMC article. Review.
Cited by
- Mutual interaction between BCL6 and microRNAs in T cell differentiation.
Wei Z, Gao W, Wu Y, Ni B, Tian Y. Wei Z, et al. RNA Biol. 2015;12(1):21-5. doi: 10.1080/15476286.2015.1017232. RNA Biol. 2015. PMID: 25826411 Free PMC article. - Charged Amino Acid-rich Leucine Zipper-1 (Crlz-1) as a Target of Wnt Signaling Pathway Controls Pre-B Cell Proliferation by Affecting Runx/CBFβ-targeted VpreB and λ5 Genes.
Choi SY, Park SK, Yoo HW, Pi JH, Kang CJ. Choi SY, et al. J Biol Chem. 2016 Jul 15;291(29):15008-19. doi: 10.1074/jbc.M115.712901. Epub 2016 May 19. J Biol Chem. 2016. PMID: 27226553 Free PMC article. - Role of B cells as antigen presenting cells.
Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Rastogi I, et al. Front Immunol. 2022 Sep 8;13:954936. doi: 10.3389/fimmu.2022.954936. eCollection 2022. Front Immunol. 2022. PMID: 36159874 Free PMC article. Review. - Central role of paxillin phosphorylation in regulation of LFA-1 integrins activity and lymphocyte migration.
Romanova LY, Mushinski JF. Romanova LY, et al. Cell Adh Migr. 2011 Nov-Dec;5(6):457-62. doi: 10.4161/cam.5.6.18219. Cell Adh Migr. 2011. PMID: 22274710 Free PMC article. Review. - Unexpected functions of nuclear factor-κB during germinal center B-cell development: implications for lymphomagenesis.
Klein U, Heise N. Klein U, et al. Curr Opin Hematol. 2015 Jul;22(4):379-87. doi: 10.1097/MOH.0000000000000160. Curr Opin Hematol. 2015. PMID: 26049760 Free PMC article. Review.
References
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. - PubMed
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. - PubMed
- Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. - PubMed
- Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007;37:100–109. - PubMed
- Balogh P, Aydar Y, Tew JG, Szakal AK. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat Rec. 2002;268:160–168. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI045073-07/AI/NIAID NIH HHS/United States
- R37 AI040098-09/AI/NIAID NIH HHS/United States
- R01 AI045073/AI/NIAID NIH HHS/United States
- R01 AI045073-08/AI/NIAID NIH HHS/United States
- R37 AI040098-08/AI/NIAID NIH HHS/United States
- R37 AI040098-10/AI/NIAID NIH HHS/United States
- R37 AI040098-11/AI/NIAID NIH HHS/United States
- R37 AI040098/AI/NIAID NIH HHS/United States
- R37 AI045073/AI/NIAID NIH HHS/United States
- R01 AI045073-09/AI/NIAID NIH HHS/United States
- R01 AI045073-06/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous