Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor - PubMed (original) (raw)
Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor
Nikhil S Joshi et al. Immunity. 2007 Aug.
Abstract
As acute infections resolve, effector CD8(+) T cells differentiate into interleukin-7 receptor(lo) (IL-7R(lo)) short-lived effector cells (SLECs) and IL-7R(hi) memory precursor effector cells (MPECs) capable of generating long-lived memory CD8(+) T cells. By using another SLEC marker, KLRG1, we found that KLRG1(hi) effector cells began appearing early during infection and were committed to downregulating IL-7R. Unlike IL-7R(hi) MPECs, KLRG1(hi) IL-7R(lo) SLECs relied on IL-15, but IL-15 could not sustain their long-term maintenance or homeostatic turnover. The decision between SLEC and MPEC fates was regulated by the amount of inflammatory cytokines (i.e., IL-12) present during T cell priming. According to the amount of inflammation, a gradient of T-bet was created in which high T-bet expression induced SLECs and low expression promoted MPECs. These results elucidate a mechanism by which the innate immune system sets the relative amounts of a lineage-determining transcription factor in activated CD8(+) T cells and, correspondingly, regulates their memory cell potential.
Figures
Figure 1
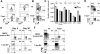
KLRG1hi IL-7Rlo effector CD8 T cells are short-lived and require IL-15 for survival. (A) IL-7Rhi and IL-7Rlo Thy1.1+ effector CD8 T cells from P14 chimeric mice were compared on day 7 of LCMV infection using Affymetrix GeneChips. Table shows the fold increase in expression for selected NK receptors (IL-7Rlo > IL-7Rhi cells). (B and C) Analysis of MPEC and SLEC subsets following LCMV infection of P14 chimeric mice. (B) Plots are gated on Thy1.1+ P14 T cells and show expression of KLRG1 and IL-7R in blood over time. (C) Line graphs show KLRG1hi IL-7Rlo (■) and KLRG1lo IL-7Rhi (▲) P14 CD8 T cell numbers in the spleen, liver, lung, inguinal lymph node (LN) and total from all tissues. The magnitude of contraction between days 8−40 and 8−75 is indicated. (D) CFSE-labeled P14 memory CD8 T cells from day ∼40 pi were transferred into naïve mice and then analyzed for CFSE and KLRG1 expression 4−6 weeks later. (E) wt and IL-15−/− mice were infected with LCMV and Db GP33−41 MHC class I tetramer+ CD8 T cells were analyzed for KLRG1 expression 8, 15 and 30 days pi. Similar data were observed for NP396−404-specific CD8 T cells (data not shown). (F) KLRG1hi IL-7Rlo or KLRG1lo IL-7Rhi P14 CD8 T cells were sorted day 8 pi and transferred in equal numbers into wt or IL-15−/− recipients for 10−15 days. Bar graph shows the number of donor cells recovered from IL-15−/− recipients normalized to the number recovered from wt recipients.
Figure 2
KLRG1 marks effector CD8 T cells committed to an SLEC fate. (A and B) P14 chimeric mice were infected with LCMV and on days 4−8 pi the Thy1.1+ P14 effector CD8 T cells were analyzed for expression of (A) KLRG1 and (B) KLRG1 and IL-7R. (B) Bottom row, histograms show IL-7R expression on KLRG1hi (filled) or KLRG1lo (open) P14 CD8 T cells. IL-7R MFI is shown (KLRG1lo/KLRG1hi). (C) Bar graph shows IL-7R mRNA levels (normalized to the ribosomal gene L9) in the indicated cell populations measured by real-time PCR. **=p<0.001. (D) Day 5 pi KLRG1hi and KLRG1lo P14 CD8 T cells were sorted and transferred in equal number back into day 5 LCMV infected recipients and analyzed 4 days post transfer (pt) for KLRG1 and IL-7R expression.
Figure 3
Phenotypic and functional comparisons between KLRG1hi and KLRG1lo effector CD8 T cells. (A) Histograms show expression of the indicated proteins on KLRG1hi (filled) and KLRG1lo (open) P14 CD8 T cells on days 5 and 8 pi. The MFI of KLRG1lo/KLRG1hi cells is shown. (B) In vivo CTL assay comparing day 5 and 8 KLRG1hi (black) and KLRG1lo (white) P14 effector CD8 T cells. The percent killing over 4 hrs was normalized to the on Effector:Target (E:T) ratio. (C) Splenocytes from day 5 and 8 LCMV infected P14 chimeric were stimulated with GP33−41 peptide and analyzed for IFNγ and IL-2 production by intracellular cytokine staining. Histograms show IFNγ (left and center) and IL-2 (right) production by KLRG1hi (filled) and KLRG1lo (open) Thy1.1+ CD8 P14 CD8 T cells. IL-2 plots are gated on IFNγ-producing cells. Similar results were found in endogenous LCMV-specific CD8 T cells stimulated with NP396−404, GP33−41 and GP276−284. Note, KLRG1 expression does not change during 5 hr stimulation (data not shown). (D) Histograms show the percent of KLRG1hi (top) and KLRG1lo cells (bottom) P14 CD8 T cells in S/G2/M phases of the cell cycle on day 5 pi using 7-AAD.
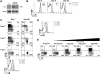
Figure 4
Levels of inflammation regulate KLRG1hi IL-7Rlo SLEC formation. (A) P14 chimeric mice were infected with LM-GP33 and 1 day pi were either left untreated (No Rx, black bars) or treated with Ampicillin (Amp Rx, white bars). FACS plots show KLRG1 and IL-7R expression and bar graphs show the number of total or KLRG1lo IL-7Rhi MPECs on day 7 pi. (B) P14 chimeric mice were infected with rVVhp −0, −17, or −19 (see text) and 8 days pi Thy1.1+ P14 CD8 T cells were analyzed for KLRG1 and IL-7R expression. (C) P14 chimeric mice were concurrently immunized with DC-33 and varying doses of Listeria (
not
expressing GP33−41). FACS plots show KLRG1 and IL-7R expression on day 7 P14 effector CD8 T cells. (D) Purified naive Thy1.1+ P14 CD8 T cells were stimulated with GP33−41 peptide-loaded cells ± CpG ODN or the indicated cytokines for 24−48h and then transferred into naïve recipients. Thy1.1+ P14 CD8 T cells were analyzed for KLRG1 and IL-7R expression 5−6 days pt. (E and F) Naïve wt or _T-bet_−/− P14 CD8 T cells were stimulated as in (D) with IL-12 or IFNγ or both (E) or decreasing concentrations of IL-12 (F). (E) Histograms show T-bet expression in wt (line) or T-bet−/− (shaded) Thy1.1+ CD44hi CD8 T cells and the T-bet MFI is indicated. (F) Line graph shows the MFI of T-bet with either peptide alone (dashed), the indicated concentration of IL-12 (solid), or _T-bet_−/− P14 CD8 T cells + IL-12 (gray). Data in graph is representative of 3 independent experiments.
Figure 5
T-bet expression is necessary and sufficient for development of KLRG1hi SLECs. (A) wt or _T-bet_−/− mice or (B) wt mice containing ∼1x104 wt or _T-bet_−/− P14 CD8 T cells were infected with LCMV and analyzed 7−8 days later for IL-7R and KLRG1 expression on GP33−41-specific CD8 T cells (A) or Thy1.1+ P14 CD8 splenocytes (B). Similar data to (A) were observed with NP396−404-specific CD8 T cells (data not shown). (C) Bar graph compares the total combined number of endogenous GP33−41 and NP396−404-specific CD8 T cells between wt (black) or _T-bet_−/− (white) animals on day 8 of LCMV infection. Note, similar numbers of IL-7Rhi effector cells in wt and _T-bet_−/− animals. **=p<0.001, *=p<0.01. (D) As in Figure 4D, wt or _T-bet_−/− Thy1.1+ P14 CD8 T cells were stimulated with IL-12, transferred into naïve recipients and analyzed for KLRG1 and IL-7R expression 5−6 days later. (E) wt P14 CD8 T cells were transduced with control (MSCV) or T-bet-expressing RVs, transferred into naïve recipients, and analyzed 5 or 30+ days later for KLRG1 and IL-7R expression on Thy1.1+ GFP+ CD8 T cells. (F) wt or _T-bet_−/− P14 CD8 T cells were transduced with MSCV or T-bet RVs and transferred into recipients that were subsequently infected with LCMV. Seven days later, Thy1.1+ GFP+ splenocytes were analyzed for IL-7R and KLRG1 expression.
Figure 6
T-bet functions in both MPECs and SLECs according to an expression gradient. (A) Naïve and day 8 KLRG1hi IL-7Rlo SLECs or KLRG1lo IL-7Rhi MPECs were sorted and examined by Western blotting T-bet and GRp94 levels. (B) wt and _T-bet_−/− P14 CD8 T cells were analyzed 5, 8 and 30 days pi for CD122 expression. (C) T-bet+/+, T-bet+/− or _T-bet_−/− P14 CD8 T cells were analyzed 7 and 30 days pi for KLRG1 and IL-7R expression and T-bet expression (bottom histogram plot). (D) T-bet+/+, T-bet+/− or _T-bet_−/− P14 memory CD8 T cells were analyzed 30 days pi for CD122 expression (E) _T-bet_−/− P14 CD8 T cells were transduced with T-bet RV or one expressing T-bet fused to the estrogen receptor (T-bet:ER) and transferred into mice subsequently infected with LCMV. Mice were treated with 0 – 8 mg of Tamoxifen (Tm) during infection and on day 7 pi, GFP+ donor splenocytes were analyzed for expression of KLRG1 and IL-7R.
Figure 7
Model of SLEC and MPEC development during acute viral infection. Naive CD8 T cells are IL-7Rhi, CD122lo (IL-2/15βR), KLRG1neg and T-betneg and are IL-7 dependent. Early during infection, most effector CD8 T cells become CD122hi and downregulate IL-7R to an intermediate-to-low level, but expression of T-bet and KLRG1 is set depending on their exposure to inflammatory cytokines (e.g. IL-12). Effector CD8 T cells that are exposed to lower levels of inflammation express less T-bet (light blue cells) and begin to upregulate IL-7R to become KLRG1lo IL-7Rhi MPECs (turquoise cells). Effector CD8 T cells that encounter higher levels of inflammatory cytokines express relatively more T-bet and KLRG1 (dark blue cells), stably repress IL-7R and consequentially become KLRG1hi IL-7Rlo SLECs. SLECs become IL-15 dependent, however, IL-15 alone cannot support their long-term persistence or homeostatic turnover and they decline over time. In contrast, MPECs remain dually responsive to IL-7 and IL-15 and preferentially develop into long-lived memory CD8 T cells that can self-renew.
Comment in
- CD8(+) T cell differentiation: choosing a path through T-bet.
Hamilton SE, Jameson SC. Hamilton SE, et al. Immunity. 2007 Aug;27(2):180-2. doi: 10.1016/j.immuni.2007.08.003. Immunity. 2007. PMID: 17723210
Similar articles
- Expression of IL-7Rα and KLRG1 defines functionally distinct CD8+ T-cell populations in humans.
Remmerswaal EBM, Hombrink P, Nota B, Pircher H, Ten Berge IJM, van Lier RAW, van Aalderen MC. Remmerswaal EBM, et al. Eur J Immunol. 2019 May;49(5):694-708. doi: 10.1002/eji.201847897. Epub 2019 Mar 25. Eur J Immunol. 2019. PMID: 30883723 Free PMC article. - IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection.
Wilson DC, Matthews S, Yap GS. Wilson DC, et al. J Immunol. 2008 May 1;180(9):5935-45. doi: 10.4049/jimmunol.180.9.5935. J Immunol. 2008. PMID: 18424713 - IL-2 induction of Blimp-1 is a key in vivo signal for CD8+ short-lived effector T cell differentiation.
Boulet S, Daudelin JF, Labrecque N. Boulet S, et al. J Immunol. 2014 Aug 15;193(4):1847-54. doi: 10.4049/jimmunol.1302365. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015830 - Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo.
Wiesel M, Crouse J, Bedenikovic G, Sutherland A, Joller N, Oxenius A. Wiesel M, et al. Eur J Immunol. 2012 Feb;42(2):320-9. doi: 10.1002/eji.201142091. Epub 2011 Dec 20. Eur J Immunol. 2012. PMID: 22102057 - Decisions on the road to memory.
Amsen D, Backer RA, Helbig C. Amsen D, et al. Adv Exp Med Biol. 2013;785:107-20. doi: 10.1007/978-1-4614-6217-0_12. Adv Exp Med Biol. 2013. PMID: 23456843 Review.
Cited by
- Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation.
Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW; Immunological Genome Project Consortium. Best JA, et al. Nat Immunol. 2013 Apr;14(4):404-12. doi: 10.1038/ni.2536. Epub 2013 Feb 10. Nat Immunol. 2013. PMID: 23396170 Free PMC article. - Early effector cells survive the contraction phase in malaria infection and generate both central and effector memory T cells.
Opata MM, Carpio VH, Ibitokou SA, Dillon BE, Obiero JM, Stephens R. Opata MM, et al. J Immunol. 2015 Jun 1;194(11):5346-54. doi: 10.4049/jimmunol.1403216. Epub 2015 Apr 24. J Immunol. 2015. PMID: 25911759 Free PMC article. - Temporary CXCR3 and CCR5 antagonism following vaccination enhances memory CD8 T cell immune responses.
Li R, Zhang N, Tian M, Ran Z, Zhu M, Zhu H, Han F, Yin J, Zhong J. Li R, et al. Mol Med. 2016 Oct;22:497-507. doi: 10.2119/molmed.2015.00218. Epub 2016 Jul 6. Mol Med. 2016. PMID: 27447731 Free PMC article. - Memory T cells are uniquely resistant to melanoma-induced suppression.
Wentworth L, Meyers JV, Alam S, Russ AJ, Suresh M, Cho CS. Wentworth L, et al. Cancer Immunol Immunother. 2013 Jan;62(1):149-59. doi: 10.1007/s00262-012-1326-1. Epub 2012 Aug 4. Cancer Immunol Immunother. 2013. PMID: 22865267 Free PMC article. - ECSIT facilitates memory CD8+ T cell development by mediating fumarate synthesis during viral infection and tumorigenesis.
Yang Y, Wang Y, Wang Z, Yan H, Gong Y, Hu Y, Jiang Y, Wen S, Xu F, Wang B, Humphries F, Chen Y, Wang X, Yang S. Yang Y, et al. Nat Cell Biol. 2024 Mar;26(3):450-463. doi: 10.1038/s41556-024-01351-9. Epub 2024 Feb 7. Nat Cell Biol. 2024. PMID: 38326554
References
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. - PubMed
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
- Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. - PubMed
- Bachmann MF, Schwarz K, Wolint P, Meijerink E, Martin S, Manolova V, Oxenius A. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI066232-01/AI/NIAID NIH HHS/United States
- R01 AI056322-01/AI/NIAID NIH HHS/United States
- R01 AI 066232-01/AI/NIAID NIH HHS/United States
- R01 AI057485/AI/NIAID NIH HHS/United States
- T32 AI055403/AI/NIAID NIH HHS/United States
- R01 AI056322/AI/NIAID NIH HHS/United States
- R01 AI057485-01/AI/NIAID NIH HHS/United States
- R01 AI054661/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P01 AI22295/AI/NIAID NIH HHS/United States
- R01 AI066232/AI/NIAID NIH HHS/United States
- P01 AI022295/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials