Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation - PubMed (original) (raw)
Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation
Xuerong Wang et al. Mol Cell Biol. 2007 Nov.
Abstract
The initiation factor eukaryotic translation initiation factor 4E (eIF4E) plays a critical role in initiating translation of mRNAs, including those encoding oncogenic proteins. Therefore, eIF4E is considered a survival protein involved in cell cycle progression, cell transformation, and apoptotic resistance. Phosphorylation of eIF4E (usually at Ser209) increases its binding affinity for the cap of mRNA and may also favor its entry into initiation complexes. Mammalian target of rapamycin (mTOR) inhibitors suppress cap-dependent translation through inhibition of the phosphorylation of eIF4E-binding protein 1. Paradoxically, we have shown that inhibition of mTOR signaling increases eIF4E phosphorylation in human cancer cells. In this study, we focused on revealing the mechanism by which mTOR inhibition increases eIF4E phosphorylation. Silencing of either mTOR or raptor could mimic mTOR inhibitors' effects to increase eIF4E phosphorylation. Moreover, knockdown of mTOR, but not rictor or p70S6K, abrogated rapamycin's ability to increase eIF4E phosphorylation. These results indicate that mTOR inhibitor-induced eIF4E phosphorylation is secondary to mTOR/raptor inhibition and independent of p70S6K. Importantly, mTOR inhibitors lost their ability to increase eIF4E phosphorylation only in cells where both Mnk1 and Mnk2 were knocked out, indicating that mTOR inhibitors increase eIF4E phosphorylation through a Mnk-dependent mechanism. Given that mTOR inhibitors failed to increase Mnk and eIF4E phosphorylation in phosphatidylinositol 3-kinase (PI3K)-deficient cells, we conclude that mTOR inhibition increases eIF4E phosphorylation through a PI3K-dependent and Mnk-mediated mechanism. In addition, we also suggest an effective therapeutic strategy for enhancing mTOR-targeted cancer therapy by cotargeting mTOR signaling and Mnk/eIF4E phosphorylation.
Figures
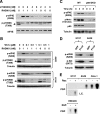
FIG. 1.
Rapamycin increases eIF4E phosphorylation under serum-free conditions (A), in the presence of IGF-1 (B) and an IGF-1R inhibitor (C), and in IRS-1-silenced (D) or -deficient (E) cells. (A) H157 cells were serum starved for 24 h and then treated with 10 nM rapamycin in the absence of FBS for the indicated times. In addition, H157 cells were cultured and treated with 10 nM rapamycin in medium containing 5% FBS for the given times. (B) H157 cells were serum starved for 20 h and then treated with 10 nM rapamycin alone, 1 ng/ml of IGF-1 alone, and their combination for 30, 60, 120, and 180 min. (C) A549 cells were pretreated with the given concentrations of IGF-1R inhibitor II (IGF-1Ri-II) for 30 min and then cotreated with 10 nM rapamycin (Rap) for the indicated times. (D) A549 cells were transfected with control (Ctrl) or IRS-1 siRNA. After 48 h, the cells were treated with 10 nM rapamycin (Rap) for 1 h before they were harvested for preparation of whole-cell protein lysates. In addition, untransfected cells (No) were included as a control. (E) WT and IRS−/− murine 3T3 fibroblasts were treated with 10 nM rapamycin (R) or RAD001 (R1) for 8 h. After the aforementioned treatments, the cells were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis.
FIG. 2.
Effects of knockdown of mTOR (A, B, and D), raptor (C and D), rictor (D), or p70S6K (E) on basal levels of eIF4E phosphorylation and rapamycin-induced eIF4E phosphorylation. (A) H157 cells were transfected with control or mTOR siRNA. After 48 h, the cells were subjected to preparation of whole-cell protein lysates. (B) H157 cells were transfected with control or mTOR siRNA for 48 h. Before the cells were harvested for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin for 3 h. (C) H157 cells were transfected with control or raptor siRNA. After the indicated times, the cells were subjected to preparation of whole-cell protein lysates. (D) Calu-1 cells were infected once with lentivirus carrying control, mTOR, raptor, or rictor shRNA and then subjected to selection with 1 μg/ml puromycin for 10 days. The surviving cells were further cultured in puromycin-free medium. After another 10 days, the cells were seeded, treated with 10 nM rapamycin for 1 h, and then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. (E) H157 cells were transfected with control or p70S6K siRNA for the indicated times. Before the cells were harvested at each time point for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin for 3 h. The indicated proteins in these experiments were detected by Western blot analysis. LE, longer exposure; Ctrl, control; TOR, mTOR; Rap, rapamycin.
FIG. 3.
Effects of Akt knockdown on eIF4E phosphorylation (A) or rapamycin-induced eIF4E phosphorylation (B). (A) H157 cells were transfected with control (Ctrl) or Akt siRNA. After 48 h, the cells were subjected to preparation of whole-cell protein lysates. (B) H157 cells were transfected with control (Ctrl) or Akt siRNA for the indicated times. Before the cells were harvested for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin (Rap) for 3 h. The indicated proteins in these experiments were detected by Western blot analysis.
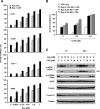
FIG. 4.
mTOR inhibitors induce PI3K-dependent eIF4E phosphorylation. (A and B) Modulation of eIF4E phosphorylation by RAD001 in the presence of the PI3K inhibitor LY294002 (A) or wortmannin (Wort.) (B). H157 (A) or H157 and A549 (B) cells were treated with 1 nM RAD001 in the absence and presence of the indicated concentrations of LY194002 (A) or wortmannin (B) for 3 h. (C) Impact of p85 deficiency on rapamycin-induced eIF4E phosphorylation. The WT and p85-DKO MEFs were treated with 10 nM rapamycin for the indicated times. (D) Effects of expressing a constitutively active p110 catalytic subunit of PI3K on eIF4E phosphorylation. The indicated cancer cell lines were plated in six-well cell culture plates and then infected with Ad-GFP or Ad-p110* for 24 h. (E) Effects of rapamycin on PI3K activity. The indicated cancer cell lines were treated with 10 nM rapamycin (Rap) for 1 h. After the aforementioned treatments, the cell lines were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for detection of the proteins as presented (A to D) or PI3K activity assay (E). L.E., longer exposure; EGF, epidermal growth factor.
FIG. 5.
mTOR inhibitors increase eIF4E phosphorylation through a Mnk-dependent mechanism. (A) Knockdown of Mnk1 does not abrogate rapamycin-induced eIF4E phosphorylation. H157 cells that were not transfected (NT) or were transfected with control (Ctrl) or Mnk1 siRNA for 48 h were treated with 10 nM rapamycin (Rap). After 3 h, the cells were subjected to preparation of whole-cell protein lysates. (B) Modulation of eIF4E phosphorylation by mTOR inhibitors in WT, 1-KO, 2-KO, and Mnk1/Mnk2 DKO cells. The indicated MEF lines were treated with 10 nM rapamycin (R) or RAD001 (R1) for 3 h and then subjected to preparation of whole-cell protein lysates. The indicated proteins in these experiments were detected by Western blot analysis.
FIG. 6.
Inhibition of Mnk-dependent eIF4E phosphorylation by a Mnk1 inhibitor (C) augments rapamycin-mediated growth inhibition (A and B) in human lung cancer cells. (A) The individual cell lines, as indicated, were seeded in 96-well plates. On the next day, they were treated with the indicated concentrations of rapamycin (Rap) alone, 2.5 μM CGP57380 (CGP) alone, and their combination. After 3 days, the plates were subjected to determination of cell numbers using the sulforhodamine B assay. The bars are means of four replicate determinations plus standard deviations. (B) H460 cells at a density of ∼ 250 cells per well were seeded in 12-well plates. On the second day, the cells were treated with the indicated concentrations of rapamycin (Rap) alone, CGP57380 (CGP) alone, and their combination. The same treatments were repeated every 3 days. After 10 days, the plates were stained for the formation of cell colonies with crystal violet. The colonies in each well were counted. The bars are means of three replicate determinations plus standard deviations. (C) H157 cells were pretreated with the indicated concentrations of CGP57380 (CGP) for 30 min and then cotreated with 10 nM rapamycin (Rap) for 1 h or 24 h. The cells were then subjected to preparation of whole-cell protein lysates for detection of the indicated proteins using Western blotting.
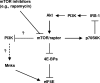
FIG. 7.
Schema for PI3K-dependent and Mnk-mediated eIF4E phosphorylation during mTOR inhibition in human cancer cells. In the literature, it has been suggested that mTOR/raptor exerts feedback inhibition of PI3K/Akt via p70S6K-mediated phosphorylation and degradation of IRS-1. Our results in this study suggest that mTOR/raptor can also negatively regulate PI3K activity through an unknown mechanism independently of p70S6K/IRS-1. Moreover, we suggest that PI3K may regulate Mnk activity. Thus, we propose that inhibition of mTOR/raptor with an mTOR inhibitor (e.g., rapamycin) can increase PI3K activity independently of p70S6K/IRS-1, resulting in a Mnk-mediated increase in eIF4E phosphorylation.
Similar articles
- Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition.
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Sun SY, et al. Cancer Res. 2005 Aug 15;65(16):7052-8. doi: 10.1158/0008-5472.CAN-05-0917. Cancer Res. 2005. PMID: 16103051 - CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway.
Wen Q, Wang W, Luo J, Chu S, Chen L, Xu L, Zang H, Alnemah MM, Ma J, Fan S. Wen Q, et al. Oncotarget. 2016 May 10;7(19):27787-801. doi: 10.18632/oncotarget.8497. Oncotarget. 2016. PMID: 27050281 Free PMC article. - Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells.
Marzec M, Liu X, Wysocka M, Rook AH, Odum N, Wasik MA. Marzec M, et al. PLoS One. 2011;6(9):e24849. doi: 10.1371/journal.pone.0024849. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949767 Free PMC article. Retracted. - Targeting Mnks for cancer therapy.
Hou J, Lam F, Proud C, Wang S. Hou J, et al. Oncotarget. 2012 Feb;3(2):118-31. doi: 10.18632/oncotarget.453. Oncotarget. 2012. PMID: 22392765 Free PMC article. Review. - Dual abrogation of MNK and mTOR: a novel therapeutic approach for the treatment of aggressive cancers.
Lineham E, Spencer J, Morley SJ. Lineham E, et al. Future Med Chem. 2017 Sep;9(13):1539-1555. doi: 10.4155/fmc-2017-0062. Epub 2017 Aug 25. Future Med Chem. 2017. PMID: 28841037 Review.
Cited by
- Lithium down-regulates histone deacetylase 1 (HDAC1) and induces degradation of mutant huntingtin.
Wu S, Zheng SD, Huang HL, Yan LC, Yin XF, Xu HN, Zhang KJ, Gui JH, Chu L, Liu XY. Wu S, et al. J Biol Chem. 2013 Dec 6;288(49):35500-10. doi: 10.1074/jbc.M113.479865. Epub 2013 Oct 28. J Biol Chem. 2013. PMID: 24165128 Free PMC article. - Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling.
Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Korkaya H, et al. PLoS Biol. 2009 Jun 2;7(6):e1000121. doi: 10.1371/journal.pbio.1000121. Epub 2009 Jun 2. PLoS Biol. 2009. PMID: 19492080 Free PMC article. - mTOR-targeted cancer therapy: great target but disappointing clinical outcomes, why?
Sun SY. Sun SY. Front Med. 2021 Apr;15(2):221-231. doi: 10.1007/s11684-020-0812-7. Epub 2020 Nov 9. Front Med. 2021. PMID: 33165737 Review. - Amiodarone's major metabolite, desethylamiodarone inhibits proliferation of B16-F10 melanoma cells and limits lung metastasis formation in an in vivo experimental model.
Bognar Z, Cseh AM, Fekete K, Antus C, Bognar R, Tapodi A, Ramadan FHJ, Sumegi B, Gallyas F Jr. Bognar Z, et al. PLoS One. 2020 Sep 25;15(9):e0239088. doi: 10.1371/journal.pone.0239088. eCollection 2020. PLoS One. 2020. PMID: 32977329 Free PMC article. - Novel targeting of phosphatidylinositol 3-kinase and mammalian target of rapamycin in renal cell carcinoma.
Cho D. Cho D. Cancer J. 2013 Jul-Aug;19(4):311-5. doi: 10.1097/PPO.0b013e31829d5cea. Cancer J. 2013. PMID: 23867512 Free PMC article. Review.
References
- Bjornsti, M. A., and P. J. Houghton. 2004. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell 5: 519-523. - PubMed
- Bjornsti, M. A., and P. J. Houghton. 2004. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer 4: 335-348. - PubMed
- De Benedetti, A., and A. L. Harris. 1999. eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell. Biol. 31: 59-72. - PubMed
- Franch, H. A., X. Wang, S. Sooparb, N. S. Brown, and J. Du. 2002. Phosphatidylinositol 3-kinase activity is required for epidermal growth factor to suppress proteolysis. J. Am. Soc. Nephrol. 13: 903-909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous