Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC - PubMed (original) (raw)
. 2007 Sep 15;120(Pt 18):3200-6.
doi: 10.1242/jcs.014902. Epub 2007 Aug 28.
Affiliations
- PMID: 17726059
- PMCID: PMC1988841
- DOI: 10.1242/jcs.014902
Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC
Scott X Atwood et al. J Cell Sci. 2007.
Abstract
Cdc42 recruits Par-6-aPKC to establish cell polarity from worms to mammals. Although Cdc42 is reported to have no function in Drosophila neuroblasts, a model for cell polarity and asymmetric cell division, we show that Cdc42 colocalizes with Par-6-aPKC at the apical cortex in a Bazooka-dependent manner, and is required for Par-6-aPKC localization. Loss of Cdc42 disrupts neuroblast polarity: cdc42 mutant neuroblasts have cytoplasmic Par-6-aPKC, and this phenotype is mimicked by neuroblast-specific expression of a dominant-negative Cdc42 protein or a Par-6 protein that lacks Cdc42-binding ability. Conversely, expression of constitutively active Cdc42 leads to ectopic Par-6-aPKC localization and corresponding cell polarity defects. Bazooka remains apically enriched in cdc42 mutants. Robust Cdc42 localization requires Par-6, indicating the presence of feedback in this pathway. In addition to regulating Par-6-aPKC localization, Cdc42 increases aPKC activity by relieving Par-6 inhibition. We conclude that Cdc42 regulates aPKC localization and activity downstream of Bazooka, thereby directing neuroblast cell polarity and asymmetric cell division.
Figures
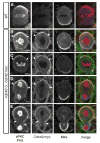
Figure 1. Cdc42 is enriched at the apical neuroblast cortex
(A) Wild-type central brain neuroblasts at 120 h after larval hatching (ALH). Normal apical and basal protein localization is shown with background c-myc staining. (B-E) cdc42-3 central brain neuroblasts at 96 h ALH expressing Cdc42:myc under its native promoter. All stages of mitosis represented. Arrowheads delineate extent of aPKC and Cdc42:myc apical crescents.
Figure 2. Cdc42 is required for neuroblast polarity
(A) Wild-type embryonic neuroblasts stages 11-13 stained for aPKC, Par-6, Baz, and Mira. (B-E) Embryonic neuroblasts stages 11-13 expressing Cdc42-DN (N17) driven by worniu-Gal4. aPKC displays ectopic cortical staining (B; 82%, n=45) along with Par-6 (C; 76%, n=41) and Mira (B′; 45%, n=67), while Baz displays no defects (D; 100%, n=26). (F) Divisions are asymmetric (100%, n=23). (F-J) Embryonic neuroblasts stages 11-13 expressing myc:Cdc42-CA (V12) as in (B-E). aPKC displays cortical, with some cytoplasmic, staining (F; 94%, n=50) along with Par-6 (G; 90%, n=29) and myc:Cdc42-CA (H; 89%, n=19), while Mira is cytoplasmic (F′; 94%, n=50). Baz displays no defects (I; 100%, n=13). (J) Neuroblast division becomes symmetric upon overexpression of Cdc42-CA (88%, n=9). (K) Wild-type central brain neuroblasts 120 h ALH stained for aPKC, Par-6, Baz, and Mira. (L-N) cdc42-3 central brain neuroblasts 96 h ALH. These neuroblasts show cytoplamsic staining of aPKC (L; 84%, n=19) and Par-6 (M; 100%, n=11), while Mira is uniformly cortical (L′-N′;100%, n=46). Baz displays no defects (N; 100%, n=16). (O) Cdc42 is mislocalized in zygotic baz-4 mutant neuroblasts. Embryonic neuroblasts stages 13-14 expressing Cdc42:myc in a baz-4 background exhibit loss of Cdc42 apical enrichment. Cdc42:myc is weakly cortical with some cytoplasmic staining and no apical enrichment (O′) whereas aPKC is cytoplasmic (O) and Mira is uniform cortical (O″; 100%, n=21). (P) Quantification of the Cdc42 requirement for neuroblast polarity in embryonic and larval neuroblasts.
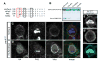
Figure 3. Cdc42/Par-6 interaction is necessary for neuroblast polarity
(A) Alignment of the Par-6 semi-CRIB domain with CRIB domains from other proteins. Mutated residues are boxed and the residues mutated in the Par-6ISAA transgene are boxed in red. (B) The ISAA mutation disrupts Cdc42 binding to the Par-6 CRIB-PDZ domain. The extent of binding between a glutathione-S-transferase (GST) fusion of GTPγS loaded Cdc42 and 55_μ_M wild-type and mutant Par-6 CRIB-PDZ domains is shown, as determined using a qualitative pull-down assay stained with coomassie brilliant blue. (C,D) Zygotic par6_Δ_226 central brain neuroblasts 24 h ALH expressing par-6 transgenes. HA:Par6 localizes to the apical cortex of dividing neuroblasts and rescues Mira phenotype (C). HA:Par-6ISAA is cytoplasmic and is unable to rescue cortical Mira (D). (E) Zygotic par6_Δ_226 central brain neuroblasts 24 h ALH expressing Cdc42:myc. Arrowhead delineates weak apical enrichment of Cdc42:myc (92%, n=12), whereas Mira is uniform cortical (100%, n=12).
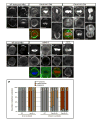
Figure 4. Par-6 represses while Cdc42 partially relieves aPKC kinase activity
(A) Kinase activity of aPKC, Par-6/aPKC, and Cdc42/Par-6/aPKC complexes. The high intrinsic kinase activity of aPKC, expressed and purified from HEK 293 cells, is efficiently repressed by addition of full-length Par-6. Par-6 has no effect on PKCα (right panel). Cdc42 partially restores aPKC activity. The signal is from a rhodamine-labeled peptide corresponding to a PKC consensus substrate (sequence shown on left). (B) aPKC fractionates predominantly with Par-6. Fractions of Drosophila embryonic lysate from stages 8-14 embryos from a calibrated gel filtration column are shown western blotted with both anti-aPKC and anti-Par-6 antibodies. Very little aPKC fractionates at its native molecular weight (~80kD), but instead co-fractionates with Par-6. (C) Pathway for regulation of apical complex activity in neuroblasts.
References
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–70. - PubMed
- Barros CS, Phelps CB, Brand AH. Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev Cell. 2003;5:829–40. - PubMed
- Beers M, Kemphues K. Depletion of the co-chaperone CDC-37 reveals two modes of PAR-6 cortical association in C. elegans embryos. Development. 2006;133:3745–54. - PubMed
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM068032/GM/NIGMS NIH HHS/United States
- T32 HD007348/HD/NICHD NIH HHS/United States
- 5-T32-HD07348/HD/NICHD NIH HHS/United States
- GM068032/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous