Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration - PubMed (original) (raw)
Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration
Solomon S Shaftel et al. J Neurosci. 2007.
Abstract
The proinflammatory cytokine interleukin-1beta (IL-1beta) plays a significant role in leukocyte recruitment to the CNS. Although acute effects of IL-1beta signaling in the mouse brain have been well described, studies elucidating the downstream effects of sustained upregulation have been lacking. Using the recently described IL-1beta(XAT) transgenic mouse model, we triggered sustained unilateral hippocampal overexpression of IL-1beta. Transgene induction led to blood-brain barrier leakage, induction of MCP-1 (monocyte chemoattractant protein 1) (CCL2), ICAM-1 (intercellular adhesion molecule 1), and dramatic infiltration of CD45-positive leukocytes comprised of neutrophils, T-cells, macrophages, and dendritic cells. Despite prolonged cellular infiltration of the hippocampus, there was no evidence of neuronal degeneration. Surprisingly, neutrophils were observed in the hippocampal parenchyma as late as 1 year after transgene induction. Their presence was coincident with upregulation of the potent neutrophil chemotactic chemokines KC (keratinocyte-derived chemokine) (CXCL1) and MIP-2 (macrophage inflammatory protein 2) (CXCL2). Knock-out of their sole receptor CXCR2 abrogated neutrophil infiltration but failed to reduce leakage of the blood-brain barrier.
Figures
Figure 1.
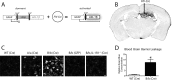
Activation of a transcriptionally silent hIL-1β transgene within the hippocampus drives leukocyte recruitment. Adult IL-1βXAT mice received intrahippocampal injections of either FIV-Cre or FIV-GFP control virus unilaterally. A, Structure of the dormant IL-1βXAT transgene and subsequent conversion to an activated state after exposure to FIV-Cre. B, A coronal section from an IL-1βXAT line B/b mouse 2 weeks after FIV-Cre injection (black arrow) into the dentate gyrus demonstrates spatially restricted CD45 staining in the ipsilateral (right) hemisphere. C, CD45 staining of leukocytes was performed in parallel groups of animals within the ipsilateral dentate gyrus represented by the white box in B. Representative images were captured from WT, IL-1βXAT line A/a (A/a), IL-1βXAT line B/b (B/b), and IL-1βXAT line B/b animals lacking IL-1R1 (B/b, IL-1R1−/−) injected with FIV encoding the protein designated in parentheses. Background CD45 staining reflects low-affinity binding to microglia within the hippocampus (scale bar, 10 μm). D, Hippocampal BBB leakage, as evidenced by Evans Blue concentration, in IL-1βXAT line B/b versus WT animals 2 weeks after transgene activation. Graph represents mean ± SEM. n = 3 animals per group. *p ≤ 0.05.
Figure 2.
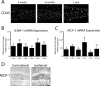
Induction of IL-1β expression drives chronic leukocyte recruitment to the mouse hippocampus. IL-1βXAT line B/b mice received unilateral intrahippocampal injections of FIV-Cre and were analyzed over a prolonged time course. A, CD45 staining in the ipsilateral (injected) hemispheres at the times indicated after FIV-Cre injection identifies infiltrating leukocytes. A white dashed line approximates the location of the granule cell layer of the dentate gyrus. qRT-PCR (B, C) generated a ratio of gene expression in the ipsilateral hippocampi of FIV-Cre injected IL-1βXAT mice compared with wild-type control animals at the same time point for ICAM-1 (B) and MCP-1 (CCL2) (C). D, Immunohistochemical detection of MCP-1 protein expression in the ipsilateral and contralateral hemispheres 2 weeks after FIV-Cre. n = 3–5 animals per time point. Scale bars: A, 25 μm; D, 50 μm. Graphs represent mean ± SEM. ns, Not significant. *p ≤ 0.05.
Figure 3.
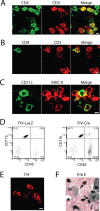
IL-1β expression recruits diverse leukocyte populations to the mouse hippocampus. Leukocyte populations were defined in the ipsilateral hippocampi of IL-1βXAT line B/b mice 2 weeks after gene activation by immunohistochemistry (A–C, E), flow cytometry (D), and tissue staining (F). A, B, T-cell populations were identified and classified by their expression of CD3 (red) and either CD4 (A; green) or CD8 (B; green), where coexpressing cells appear yellow in merged images. C, The presence of dendritic cells was established by expression of both CD11c (green) and MHC-II (red), appearing yellow in merged images. D, An increase in the number of infiltrating macrophages is demonstrated 2 weeks after FIV-Cre versus FIV-LacZ injection by expansion of the CD45hi, CD11b+ cell population. E, F, Neutrophils were identified by cellular labeling with the 7/4 antibody (E) and after hematoxylin and eosin (H&E) tissue staining (F, arrows). N, Neuronal nuclei. Scale bars, 5 μm.
Figure 4.
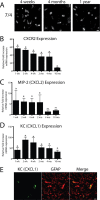
IL-1β transgene activation leads to sustained neutrophil recruitment to the hippocampus. A, Neutrophils were identified in the parenchyma of the dentate gyrus using the 7/4 antibody at all time points assayed. Using qRT-PCR analysis (B–D), we determined relative gene expression in the ipsilateral hippocampi of FIV-Cre-injected IL-1βXAT line B/b versus WT mice at the same time point. B, CXCR2 expression was used as a surrogate marker for neutrophil infiltration and was significantly upregulated in the hippocampi of IL-1βXAT mice at all time points analyzed. C, MIP-2 (CXCL2) expression was significantly upregulated only until 2 months after transgene activation. D, Significant, robust overexpression of the neutrophil chemotactic chemokine KC (CXCL1) was also detected at all time points. E, Expression of KC (green) colocalizes with GFAP (red), appearing yellow in the ipsilateral hippocampus of an IL-1βXAT line B/b mouse 4 weeks after FIV-Cre injection. n = 3–5 animals per time point. Scale bars, 10 μm. Graphs represent mean ± SEM. ns, Not significant. *p ≤ 0.05.
Figure 5.
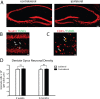
Neuronal toxicity is not evident after sustained hippocampal IL-1β expression. IL-1βXAT line B/b mice received FIV-Cre injections and were analyzed 2 weeks or 2 months thereafter. Determination of neuronal toxicity was made comparing ipsilateral (injected) to noninjected (contralateral) hippocampi within animals. A, Preservation of both the pyramidal and granule cell layers of the hippocampus is demonstrated using NeuN (red) in the ipsilateral and contralateral hippocampi 2 weeks after FIV-Cre injection. B, NeuN (red) and TUNEL+ cells (green, white arrows) 2 weeks after FIV-Cre injection. No overlap between these markers was detected. C, Leukocytes undergoing apoptosis (yellow, indicated by white arrows) expressed both TUNEL (green) and CD45 (red) markers 2 weeks after FIV-Cre injection. D, Determination of granule cell density within the dentate gyrus of animals 2 weeks and 2 months after FIV-Cre injection. Statistical analysis compared the ipsilateral versus contralateral cellular density between groups of animals at the same time point. n = 3–4 animals per time point. Scale bars: A, 100 μm; B, 25 μm; C, 10 μm. n = 3–4 animals per time point. Graphs represent mean ± SEM. ns, Not significant.
Figure 6.
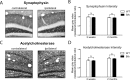
IL-1β transgene activation does not cause changes in neuronal integrity. A, Synaptophysin staining in the contralateral and ipsilateral dentate gyrus of an IL-1βXAT mouse 2 months after transgene activation. A rectangular box in the molecular layer indicates the region of quantitative analysis. B, Quantification of synaptophysin staining in WT and IL-1βXAT B/b mice (B/b) 2 weeks and 2 months after FIV-Cre injection. Statistical analysis compared the ratio of staining intensity between the ipsilateral and contralateral hippocampi of B/b and WT animals at the same time point. C, D, Acetylcholinesterase staining (C) and quantification (D) as described in A and B above. n = 3–5 animals per time point, 1–3 sections per animal analyzed. Scale bars, 50 μm. Graphs represent mean ± SEM. ns, Not significant.
Figure 7.
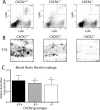
CXCR2 is required for neutrophil infiltration after hippocampal IL-1β expression. IL-1βXAT line B/b animals either lacking (CXCR2−/−), heterozygous (CXCR2+/−), or with two copies (CXCR2+/+) of CXCR2 were examined 2 weeks after FIV-Cre hippocampal injections. A, Hippocampal cellular infiltrates from bilaterally injected mice were analyzed by flow cytometry. After gating on M-CSF-negative cells, the percentage of neutrophils are indicated in the top right corner of each plot as the Lys6Ghi, Lys6Chi cell population. B, 7/4 staining of neutrophils within the dentate gyrus reflects the results from A. C, BBB leakage was determined using hippocampal Evans Blue concentrations and is graphed relative to that observed in CXCR2+/+ animals. BBB leakage was present in all animal groups and was not significantly altered by CXCR2 gene dosage. n = 3–4 animals per group. Scale bar, 20 μm. Graphs represent mean ± SEM. ns, Not significant.
Similar articles
- CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses.
Kielian T, Barry B, Hickey WF. Kielian T, et al. J Immunol. 2001 Apr 1;166(7):4634-43. doi: 10.4049/jimmunol.166.7.4634. J Immunol. 2001. PMID: 11254722 - Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation.
Zhou H, Andonegui G, Wong CH, Kubes P. Zhou H, et al. J Immunol. 2009 Oct 15;183(8):5244-50. doi: 10.4049/jimmunol.0901309. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786543 - Role of cytokines in photodynamic therapy-induced local and systemic inflammation.
Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Gollnick SO, et al. Br J Cancer. 2003 Jun 2;88(11):1772-9. doi: 10.1038/sj.bjc.6600864. Br J Cancer. 2003. PMID: 12771994 Free PMC article. - Involvement of KC, MIP-2, and MCP-1 in leukocyte infiltration following injection of necrotic cells into the peritoneal cavity.
Tanimoto N, Terasawa M, Nakamura M, Kegai D, Aoshima N, Kobayashi Y, Nagata K. Tanimoto N, et al. Biochem Biophys Res Commun. 2007 Sep 21;361(2):533-6. doi: 10.1016/j.bbrc.2007.07.060. Epub 2007 Jul 23. Biochem Biophys Res Commun. 2007. PMID: 17662241 - Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks.
Semple BD, Kossmann T, Morganti-Kossmann MC. Semple BD, et al. J Cereb Blood Flow Metab. 2010 Mar;30(3):459-73. doi: 10.1038/jcbfm.2009.240. Epub 2009 Nov 11. J Cereb Blood Flow Metab. 2010. PMID: 19904283 Free PMC article. Review.
Cited by
- Time and age dependent regulation of neuroinflammation in a rat model of mesial temporal lobe epilepsy: Correlation with human data.
Erisken S, Nune G, Chung H, Kang JW, Koh S. Erisken S, et al. Front Cell Dev Biol. 2022 Sep 13;10:969364. doi: 10.3389/fcell.2022.969364. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172274 Free PMC article. - Major Depression: One Brain, One Disease, One Set of Intertwined Processes.
Filatova EV, Shadrina MI, Slominsky PA. Filatova EV, et al. Cells. 2021 May 21;10(6):1283. doi: 10.3390/cells10061283. Cells. 2021. PMID: 34064233 Free PMC article. Review. - Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model.
Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, O'Banion MK. Ghosh S, et al. J Neurosci. 2013 Mar 13;33(11):5053-64. doi: 10.1523/JNEUROSCI.4361-12.2013. J Neurosci. 2013. PMID: 23486975 Free PMC article. - The role of neutrophils in neuro-immune modulation.
Kanashiro A, Hiroki CH, da Fonseca DM, Birbrair A, Ferreira RG, Bassi GS, Fonseca MD, Kusuda R, Cebinelli GCM, da Silva KP, Wanderley CW, Menezes GB, Alves-Fiho JC, Oliveira AG, Cunha TM, Pupo AS, Ulloa L, Cunha FQ. Kanashiro A, et al. Pharmacol Res. 2020 Jan;151:104580. doi: 10.1016/j.phrs.2019.104580. Epub 2019 Nov 28. Pharmacol Res. 2020. PMID: 31786317 Free PMC article. Review. - In-depth characterization of the neuroinflammatory reaction induced by peripheral surgery in an animal model.
Plaschke K, Schulz S, Rullof R, Weigand MA, Kopitz J. Plaschke K, et al. J Neural Transm (Vienna). 2018 Oct;125(10):1487-1494. doi: 10.1007/s00702-018-1909-x. Epub 2018 Jul 23. J Neural Transm (Vienna). 2018. PMID: 30039507
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. - PubMed
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120:435–444. - PubMed
- Bell MD, Taub DD, Perry VH. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74:283–292. - PubMed
- Bennett JL, Elhofy A, Canto MC, Tani M, Ransohoff RM, Karpus WJ. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45highCD11b+ monocytes and enhanced Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Neurovirol. 2003;9:623–636. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS033553/NS/NINDS NIH HHS/United States
- R21 NS048522/NS/NINDS NIH HHS/United States
- R29 NS033553/NS/NINDS NIH HHS/United States
- T32 GM007356/GM/NIGMS NIH HHS/United States
- NS048522/NS/NINDS NIH HHS/United States
- NS33553/NS/NINDS NIH HHS/United States
- GM07356/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous