Fatty acid chain-elongation in perfused rat heart: synthesis of stearoylcarnitine from perfused palmitate - PubMed (original) (raw)
Fatty acid chain-elongation in perfused rat heart: synthesis of stearoylcarnitine from perfused palmitate
Janos Kerner et al. FEBS Lett. 2007.
Abstract
Rat hearts perfused for up to 60 min in the working mode with palmitate, but not with glucose, resulted in substantial formation of palmitoylcarnitine and stearoylcarnitine. To test whether lipolysis of endogenous lipids was responsible for the increased stearoylcarnitine content or whether some of the perfused palmitate underwent chain elongation, hearts were perfused with hexadecanoic-16,16,16-d(3) acid (M+3). The pentafluorophenacyl ester of deuterium labeled stearoylcarnitine had an M+3 (639.4 m/z) compared to the unlabeled M+0 (636.3 m/z) consistent with a direct chain elongation of the perfused palmitate. Furthermore, the near equal isotope enrichment of palmitoyl- (90.2+/-5.8%) and stearoylcarnitine (78.0+/-7.1%) suggest that both palmitoyl- and stearoyl-CoA have ready access to mitochondrial carnitine palmitoyltransferase and that most of the stearoylcarnitine is derived from the perfused palmitate.
Figures
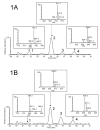
Figure 1
HPLC separation and mass spectrometric analysis of long-chain acylcarnitines extracted from rat hearts perfused for 60 minutes with 16,16,16-_d_3 palmitate (M+3) (1A) and unlabeled palmitate (M+0) (1B). Peak labeling: myristoylcarnitine (1); palmitoylcarnitine (2); heptadecanoylcarnitine - internal standard (3); stearoylcarnitine (4). The inserts in 1A and 1B show the mass isotopomer distribution of unlabeled (M+0) and deuterium labeled (M+3) pentaflourophenacyl esters of myristoylcarnitine (580.2; 583.3), palmitoylcarnitine (608.3; 611.3), and stearoylcarnitine (636.3; 639.4), respectively.
Similar articles
- Fatty acid chain elongation in palmitate-perfused working rat heart: mitochondrial acetyl-CoA is the source of two-carbon units for chain elongation.
Kerner J, Minkler PE, Lesnefsky EJ, Hoppel CL. Kerner J, et al. J Biol Chem. 2014 Apr 4;289(14):10223-34. doi: 10.1074/jbc.M113.524314. Epub 2014 Feb 20. J Biol Chem. 2014. PMID: 24558043 Free PMC article. - Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart.
Broderick TL, Quinney HA, Lopaschuk GD. Broderick TL, et al. J Biol Chem. 1992 Feb 25;267(6):3758-63. J Biol Chem. 1992. PMID: 1740427 - Glucose oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats.
Wall SR, Lopaschuk GD. Wall SR, et al. Biochim Biophys Acta. 1989 Nov 6;1006(1):97-103. doi: 10.1016/0005-2760(89)90328-7. Biochim Biophys Acta. 1989. PMID: 2804076 - Fatty acid metabolism in hearts containing elevated levels of CoA.
Lopaschuk GD, Hansen CA, Neely JR. Lopaschuk GD, et al. Am J Physiol. 1986 Mar;250(3 Pt 2):H351-9. doi: 10.1152/ajpheart.1986.250.3.H351. Am J Physiol. 1986. PMID: 3953832 - Implications of thiolester linked fatty acids in apolipoprotein B.
Lee DM. Lee DM. Adv Exp Med Biol. 1991;285:49-58. doi: 10.1007/978-1-4684-5904-3_5. Adv Exp Med Biol. 1991. PMID: 1858576 Review. No abstract available.
Cited by
- Rewired metabolism in drug-resistant leukemia cells: a metabolic switch hallmarked by reduced dependence on exogenous glutamine.
Stäubert C, Bhuiyan H, Lindahl A, Broom OJ, Zhu Y, Islam S, Linnarsson S, Lehtiö J, Nordström A. Stäubert C, et al. J Biol Chem. 2015 Mar 27;290(13):8348-59. doi: 10.1074/jbc.M114.618769. Epub 2015 Feb 19. J Biol Chem. 2015. PMID: 25697355 Free PMC article. - Recent progress in cryopreservation of bovine oocytes.
Hwang IS, Hochi S. Hwang IS, et al. Biomed Res Int. 2014;2014:570647. doi: 10.1155/2014/570647. Epub 2014 Mar 16. Biomed Res Int. 2014. PMID: 24738063 Free PMC article. - Mitochondria in the elderly: Is acetylcarnitine a rejuvenator?
Rosca MG, Lemieux H, Hoppel CL. Rosca MG, et al. Adv Drug Deliv Rev. 2009 Nov 30;61(14):1332-1342. doi: 10.1016/j.addr.2009.06.009. Epub 2009 Aug 29. Adv Drug Deliv Rev. 2009. PMID: 19720100 Free PMC article. Review. - Fatty acid chain elongation in palmitate-perfused working rat heart: mitochondrial acetyl-CoA is the source of two-carbon units for chain elongation.
Kerner J, Minkler PE, Lesnefsky EJ, Hoppel CL. Kerner J, et al. J Biol Chem. 2014 Apr 4;289(14):10223-34. doi: 10.1074/jbc.M113.524314. Epub 2014 Feb 20. J Biol Chem. 2014. PMID: 24558043 Free PMC article. - Quantitative acylcarnitine determination by UHPLC-MS/MS--Going beyond tandem MS acylcarnitine "profiles".
Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Minkler PE, et al. Mol Genet Metab. 2015 Dec;116(4):231-41. doi: 10.1016/j.ymgme.2015.10.002. Epub 2015 Oct 8. Mol Genet Metab. 2015. PMID: 26458767 Free PMC article.
References
- Hagve TA, Sprecher H. Metabolism of long-chain polyunsaturated fatty acids in isolated cardiac myocytes. Biochim Biophys Acta. 1989;1001:338–344. - PubMed
- Jimenez Lopez JA, Bordoni A, Lorenzini A, Rossi CA, Biagi PL, Hrelia S. Linoleic acid metabolism in primary cultures of adult rat cardiomyocytes is impaired by aging. Biochem Biophys Res Commun. 1997;237:142–145. - PubMed
- Ford DA, Han X, Horner CC, Gross RW. Accumulation of unsaturated acylcarnitine molecular species during acute myocardial ischemia: metabolic compartmentalization of products of fatty acyl chain elongation in the acylcarnitine pool. Biochemistry. 1996;35:7903–7909. - PubMed
- Kudo N, Barr AJ, Barr RL, Desaii S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–1752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources