The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation - PubMed (original) (raw)
The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation
Narendra Pathak et al. Mol Biol Cell. 2007 Nov.
Abstract
Cilia and basal bodies are essential organelles for a broad spectrum of functions, including the development of left-right asymmetry, kidney function, cerebrospinal fluid transport, generation of photoreceptor outer segments, and hedgehog signaling. Zebrafish fleer (flr) mutants exhibit kidney cysts, randomized left-right asymmetry, hydrocephalus, and rod outer segment defects, suggesting a pleiotropic defect in ciliogenesis. Positional cloning flr identified a tetratricopeptide repeat protein homologous to the Caenorhabditis elegans protein DYF1 that was highly expressed in ciliated cells. flr pronephric cilia were shortened and showed a reduced beat amplitude, and olfactory cilia were absent in mutants. flr cilia exhibited ultrastructural defects in microtubule B-tubules, similar to axonemes that lack tubulin posttranslational modifications (polyglutamylation or polyglycylation). flr cilia showed a dramatic reduction in cilia polyglutamylated tubulin, indicating that flr encodes a novel modulator of tubulin polyglutamylation. We also found that the C. elegans flr homologue, dyf-1, is also required for tubulin polyglutamylation in sensory neuron cilia. Knockdown of zebrafish Ttll6, a tubulin polyglutamylase, specifically eliminated tubulin polyglutamylation and cilia formation in olfactory placodes, similar to flr mutants. These results are the first in vivo evidence that tubulin polyglutamylation is required for vertebrate cilia motility and structure, and, when compromised, results in failed ciliogenesis.
Figures
Figure 1.
The fleer mutant phenotype. (A) Wild-type embryo at 56 hpf. (B) flr mutant embryo exhibiting ventral axis curvature, hydrocephalus (arrowhead), and kidney cysts (arrow). (C) Histological section of normal pronephric kidney at 72 hpf (gl, glomerulus). (D) flr mutant kidney exhibiting cystic distension (asterisk) and expanded capillary loops (arrow; gl, glomerulus). (E) Electron micrograph of the photoreceptor cell layer of a 84-hpf larva showing rod cells and their outer segments (os) beneath the retinal pigmented epithelium. (n, nucleus; m, mitochondria). (F) flr mutant retina specifically lack rod outer segments.
Figure 2.
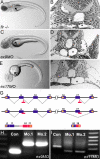
Positional cloning and characterization of fleer mRNAs. (A) flr is located on chromosome 3, tightly linked to flanking SSR markers z10805 (1/578 meioses) and z63912 (1/1450 meioses). BAC zC76L23, located within the flr interval, contained a predicted TPR protein that is present in multiple cilia proteome databases and thus a potential flr candidate. (B) cDNA sequence of the flr candidate from WT and _flr_−/− embryos identified a T (798) > A nonsense mutation (arrowhead) that replaces a wild-type tyrosine (266) with an ochre stop codon. (C) The flr gene contains 19 exons which encode two mRNA isoforms. In flr_tv1, an upstream splice donor is used in exon 12 and exon 17 is omitted. (D) Fleer and Fleer_tv1 are predicted to contain four TPR motifs and a coiled-coil domain (CC). (E) Alignment of predicted Fleer related proteins from invertebrate and vertebrate species demonstrate evolutionary conservation. Red indicates identity, and yellow shading outlines conserved TPR domains.
Figure 3.
Antisense morpholinos targeting the fleer candidate phenocopy the flr m477 mutant. (A) _flr_−/− embryos exhibit axis curvature and bilateral pronephric cysts (arrows). (B) Histological section of flr mutant showing tangential section of pronephric cyst (asterisk). (C) Embryos injected with morpholinos targeting the splice donor sites in flr exon 9 exhibit kidney cysts and axis curvature identical to flr m477. (D) Histology of the pronephric region in flr exon 9 morphant showing cyst formation. (E) Exon 17 morphants show cystic distention (asterisk) within the pronephros. (G) Summary of molecular defects caused by morpholino targeting of splice donor sites at exon 9 and 17. (H) RT-PCR analysis using primers flanking the exon 9 show an increase in amplicon size due to inclusion of intron 9 in the altered mRNA. (I) RT-PCR analysis using primers flanking the exon 17 shows a decrease in amplicon size due to exon 17 skipping. Analysis of two different single morphant larvae for each morpholino demonstrates reproducibility of flr knockdown.
Figure 4.
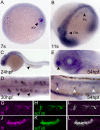
Expression of fleer mRNA and protein in ciliated epithelial cells. (A) Whole mount in situ hybridization in seven somite-stage embryos demonstrated flr expression in Kupffer's vesicle cells (arrowhead; KV). (B) At the 11-somite stage, flr expression is detected in the intermediate mesoderm that will form the pronephros (arrowhead; Pn) and the otic vesicle (arrowhead; Otc). (C) At 24 hpf, pronephric expression persists in differentiated epithelial cells (arrowhead). (D) flr expression is highest at 30 hpf in multiciliated cells of the pronephros (arrowheads) (E) At 54 hpf, flr is expressed in the olfactory placode (arrowhead). (F) Lateral line organs are also positive for flr mRNA at 54 hpf (white arrowheads). (G–L) Colocalization of Fleer and acetylated tubulin in cilia. Whole mount double immunofluorescence with anti-Fleer (G) and anti-acetylated tubulin (H) in lumenal cilia of the pronephros. (I) Merge of G and H. Expression of Fleer (J) and acetylated tubulin (K) in cilia that ring the olfactory placode. (L) Merge of J and K.
Figure 5.
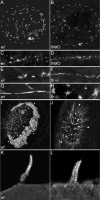
flr loss of function differentially affects cilia in different organs. Cilia were visualized in wild-type and flr mutant or morphant larvae stained with an antibody to acetylated tubulin. (A) Kupffer's vesicle contains radially arranged cilia of uniform length in wild-type embryos. White dashed outline represents the borders of Kupffer's vesicle in A and B. (B) flr morphant embryos exhibit severe shortening or absence of cilia preferentially at the periphery of Kupffer's vesicle. (C) In the presumptive pronephric duct at the 10-somite stage, short cilia were apparent in both wild-type (C) and flr morphant (D) embryos. (E) Later, at 48 hpf, single cilia and multiciliated cells are observed in wild-type embryos, whereas in the flr mutant pronephros, (F) cilia were shorter and multiciliated cells were not observed. (G) In 88-hpf wild-type larvae, multiciliated cells project dense, brightly staining bundles of cilia into the narrow lumen of the pronephros. (H) flr mutant pronephroi at 88 hpf contain dispersed bundles of cilia that are nonuniform in length. (I) The dense ring of cilia on the epithelium surrounding the olfactory placode (arrowheads) of 60-hpf wild-type larvae was absent in 60h flr mutant larvae (J). Residual staining for acetylated tubulin in flr mutant olfactory placodes was associated with intracellular projections of olfactory neurons. (K) Lateral line organ cilia project from the skin of a 96-hpf larva. (L) flr mutant lateral line organ cilia at 96 hpf are shorter and slightly dispersed.
Figure 6.
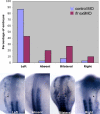
fleer loss of function results in defects of left-right asymmetry. In situ hybridization of wild-type and flr morphant embryos with southpaw probe. Nearly, all wild-type embryos showed normal left-sided expression at the 18-somite stage. flr exon 9 morphants exhibited randomization of the left-right axis with 44% left-sided expression, 20% absent expression, 26% bilateral expression, and 10% right-sided expression (top). Examples of southpaw expression patterns (bottom) showing normal left-sided expression or altered absent, bilateral, or right-sided expression.
Figure 7.
fleer loss of function causes structural defects in the ciliary axoneme. (A) Ultrastructure of normal single pronephric cilia showing 9 + 2 microtubule architecture at 56 hpf and intact B-tubules of the microtubule doublets (arrowheads). (B) flr mutant cilia in cross section show a specific loss of structure in the outer aspect of the B-tubules (arrowheads), with gaps in microtubules and accumulation of some electron-dense material between the doublets and the cilia outer membrane.
Figure 8.
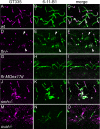
fleer is required for axonemal tubulin polyglutamylation. Tubulin polyglutamylation and acetylation was visualized in pronephric cilia by sequential labeling with antibodies mAb GT335 and mAb 6-11B-1. (A) In wild-type pronephric cilia at 56 hpf, polyglutamylated tubulin is distributed in a decreasing gradient from the basal body to the cilia tip. (B) Acetylated tubulin immunostaining of wild-type cilia reveals the total length of the cilia. (C) Merge of A and B. (D) In flr mutant pronephric cilia, polyglutamylated tubulin is restricted to the basal body region and severely reduced in axonemes. (E) Acetylated tubulin staining could still be observed in axonemes devoid of the polygutamylated tubulin. (F) Merge of D and E shows that polyglutamylated tubulin is found only in the proximal segment of flr cilia. (G) flr exon 17 morphants also show a dramatic reduction in polyglutamylated tubulin, whereas overall cilia length (H) is only slightly affected. (I) Merge of G and H shows restriction of the polyglutamylated tubulin to the basal body region in flr morphants. (J) The cystic mutant schmalhans shows normal tubulin polyglutamylation and cilia length (K). (L) Merge of J and K. (M) The TPR protein mutant oval/ift88 shows polyglutamylation extending to the tips of cilia. (N) Cilia are shortened in oval/ift88 mutants; however, the merged anti-polyglutamylated tubulin/acetylated tubulin image (O) shows a relatively normal pattern of polyglutamylation.
Figure 9.
Loss of tubulin polyglutamylation in dyf-1 C. elegans mutant cilia. Tubulin polyglutamylation in C. elegans hermaphrodite sensory cilia was detected with the mAb GT335 (magenta) and overall cilia structure was detected with anti-OSM-5 antibody (green). Anti-OSM-5 staining also gave nonspecific labeling of the pharynx, unrelated to cilia. (A) GT335 immunoreactivity identifies polyglutamylated tubulin in multiple wild-type hermaphrodite cilia: outer labial (large arrowheads; ol), inner labial (small arrowheads; il) and amphid cilia (small arrow; a). (B) OSM-5 positive outer labial cilia (arrowheads). (C) Merge of A and B; arrowheads denote outer labial cilia positive for both GT335 and OSM-5. (D) Polyglutamylated tubulin in dyf-1 mutant hermaphrodites is reduced. Large arrowheads, position of outer labial cilia; small arrow, amphid cilia. (E) OSM-5–positive outer labial cilia in dyf-1 mutants. (F) Merge of D and E; dyf-1 outer labial cilia are negative for GT335. (G) Polyglutamylated tubulin is unaffected in osm-3 mutant hermaphrodite cilia. Large arrowheads, outer labial cilia. (H) OSM-5 positive outer labial cilia are present in osm-3 mutants. (I) Merge of G and H; GT335 and OSM-5 colocalize in osm-3 mutant outer labial cilia (large arrowheads).
Figure 10.
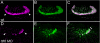
Knockdown of ttll6 polyglutamylase prevents cilia formation and polyglutamylation in the olfactory placode. (A) Olfactory cilia are polyglutamylated. GT335 immunoreactivity is strong in olfactory multiciliated cells (arrowheads). (B) Acetylated tubulin staining identifies all olfactory cilia. (C) Merge of A and B; olfactory cilia are uniformly polyglutamylated. (D) Knockdown of ttll6 polyglutamylase severely reduces GT335 immunoreactivity in the position of olfactory cilia (arrowheads). (E) ttll6 knockdown severely reduces cilia number and length as indicated by immunostaining for acetylated tubulin. (F) Merge of D and E; in a small tuft of remnant cilia, polyglutamylation of tubulin is restricted to the basal body region of cilia, and it does not extend to the axonemes (double arrows).
Figure 11.
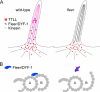
Hypothetical models of Fleer/DYF-1 function. (A) Fleer/DYF-1 could act as an adaptor protein that links TTLL enzymes to kinesins and intraflagellar transport. Anterograde transport of TTLL enzymes favors tubulin polyglutamylation all along the length of the axoneme (red microtubules). In the absence of Fleer/DYF-1, tubulin is polyglutamylated only in the basal body/basal region of the cilia by local diffusion of TTLL enzymes and distal microtubule doublets are not polyglutamylated (black microtubules). (B) Alternatively, Fleer/DYF-1 could play a structural role in maintaining the connection between B-tubule microtubule protofilaments and the A-subfiber of cilia doublets. In the absence of Fleer/DYF-1, structural defects in cilia microtubule doublets could interfere with intraflagellar transport.
Similar articles
- CSAP localizes to polyglutamylated microtubules and promotes proper cilia function and zebrafish development.
Backer CB, Gutzman JH, Pearson CG, Cheeseman IM. Backer CB, et al. Mol Biol Cell. 2012 Jun;23(11):2122-30. doi: 10.1091/mbc.E11-11-0931. Epub 2012 Apr 4. Mol Biol Cell. 2012. PMID: 22493317 Free PMC article. - Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility.
Pathak N, Austin CA, Drummond IA. Pathak N, et al. J Biol Chem. 2011 Apr 1;286(13):11685-95. doi: 10.1074/jbc.M110.209817. Epub 2011 Jan 24. J Biol Chem. 2011. PMID: 21262966 Free PMC article. - The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans.
O'Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KC, Hall DH, Swoboda P, Barr MM. O'Hagan R, et al. Curr Biol. 2011 Oct 25;21(20):1685-94. doi: 10.1016/j.cub.2011.08.049. Epub 2011 Oct 6. Curr Biol. 2011. PMID: 21982591 Free PMC article. - Polyglutamylation and the fleer gene.
Pathak NH, Drummond IA. Pathak NH, et al. Methods Cell Biol. 2009;94:317-32. doi: 10.1016/S0091-679X(08)94016-4. Epub 2009 Dec 23. Methods Cell Biol. 2009. PMID: 20362098 Review. No abstract available. - Tubulin polyglutamylation, a regulator of microtubule functions, can cause neurodegeneration.
Bodakuntla S, Janke C, Magiera MM. Bodakuntla S, et al. Neurosci Lett. 2021 Feb 16;746:135656. doi: 10.1016/j.neulet.2021.135656. Epub 2021 Jan 19. Neurosci Lett. 2021. PMID: 33482309 Review.
Cited by
- Zebrafish: a vertebrate tool for studying basal body biogenesis, structure, and function.
Marshall RA, Osborn DP. Marshall RA, et al. Cilia. 2016 May 10;5:16. doi: 10.1186/s13630-016-0036-2. eCollection 2016. Cilia. 2016. PMID: 27168933 Free PMC article. Review. - Congenital hydrocephalus: a review of recent advances in genetic etiology and molecular mechanisms.
Liu XY, Song X, Czosnyka M, Robba C, Czosnyka Z, Summers JL, Yu HJ, Gao GY, Smielewski P, Guo F, Pang MJ, Ming D. Liu XY, et al. Mil Med Res. 2024 Aug 12;11(1):54. doi: 10.1186/s40779-024-00560-5. Mil Med Res. 2024. PMID: 39135208 Free PMC article. Review. - Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules.
He K, Ma X, Xu T, Li Y, Hodge A, Zhang Q, Torline J, Huang Y, Zhao J, Ling K, Hu J. He K, et al. Nat Commun. 2018 Aug 17;9(1):3310. doi: 10.1038/s41467-018-05867-1. Nat Commun. 2018. PMID: 30120249 Free PMC article. - Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish.
Kim HR, Richardson J, van Eeden F, Ingham PW. Kim HR, et al. BMC Biol. 2010 Apr 19;8:65. doi: 10.1186/1741-7007-8-65. BMC Biol. 2010. PMID: 20487519 Free PMC article. - Robust interaction of IFT70 with IFT52-IFT88 in the IFT-B complex is required for ciliogenesis.
Takei R, Katoh Y, Nakayama K. Takei R, et al. Biol Open. 2018 Apr 30;7(5):bio033241. doi: 10.1242/bio.033241. Biol Open. 2018. PMID: 29654116 Free PMC article.
References
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. - PubMed
- Avidor-Reiss T., Maer A. M., Koundakjian E., Polyanovsky A., Keil T., Subramaniam S., Zuker C. S. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. - PubMed
- Bisgrove B. W., Snarr B. S., Emrazian A., Yost H. J. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev. Biol. 2005;287:274–288. - PubMed
- Blacque O. E., et al. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 2005;15:935–941. - PubMed
- Brand M., et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK054711/DK/NIDDK NIH HHS/United States
- DK-53093/DK/NIDDK NIH HHS/United States
- R01 DK078209/DK/NIDDK NIH HHS/United States
- T32-DK007540-20/DK/NIDDK NIH HHS/United States
- DK-54711/DK/NIDDK NIH HHS/United States
- R01 DK053093/DK/NIDDK NIH HHS/United States
- R21 DK069604/DK/NIDDK NIH HHS/United States
- T32 DK007540/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases