Congenital bone fractures in spinal muscular atrophy: functional role for SMN protein in bone remodeling - PubMed (original) (raw)
Review
Congenital bone fractures in spinal muscular atrophy: functional role for SMN protein in bone remodeling
Srinivasan Shanmugarajan et al. J Child Neurol. 2007 Aug.
Abstract
Spinal muscular atrophy is the second most common fatal childhood disorder. Core clinical features include muscle weakness caused by degenerating lower motor neurons and a high incidence of bone fractures and hypercalcemia. Fractures further compromise quality of life by progression of joint contractures or additional loss of motor function. Recent observations suggest that bone disease in spinal muscular atrophy may not be attributed entirely to lower motor neuron degeneration. The presence of the spinal muscular atrophy disease-determining survival motor neuron gene (SMN), SMN expression, and differential splicing in bone-resorbing osteoclasts was recently discovered. Its ubiquitous expression and the differential expression of splice variants suggest that SMN has specific roles in bone cell function. SMN protein also interacts with osteoclast stimulatory factor. Mouse models of human spinal muscular atrophy disease suggest a potential role of SMN protein in skeletal development. Dual energy x-ray absorptiometry analysis demonstrated a substantial decrease in total bone area and poorly developed caudal vertebra in the mouse model. These mice also had pelvic bone fractures. Studies delineating SMN signaling mechanisms and gene transcription in a cell-specific manner will provide important molecular insights into the pathogenesis of bone disease in children with spinal muscular atrophy. Moreover, understanding bone remodeling in spinal muscular atrophy may lead to novel therapeutic approaches to enhance skeletal health and quality of life. This article reviews the skeletal complications associated with spinal muscular atrophy and describes a functional role for SMN protein in osteoclast development and bone resorption activity.
Figures
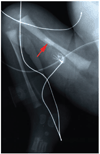
Figure 1
The arrow indicates a bone fracture in a newborn with spinal muscular atrophy. The radiograph is courtesy of the University of Miami and the University of Maryland Brain and Tissue Bank for Developmental Disorders.
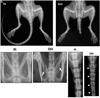
Figure 2
Skeletal phenotype in Smn−/−SMN2 mice. (A) Radiologic assessment of 4-week-old Smn−/−SMN2 (Jackson Laboratories, Bar Harbor, ME) mice shows short tails compared with wild-type mice. A high-resolution (3x) radiogram indicates pelvic bone fractures and poorly developed caudal vertebra in spinal muscular atrophy mice. (B) Dual energy x-ray absorptiometry analyses of total animal bone area and total spine for bone area, bone mineral content, and bone mineral density in spinal muscular atrophy mice. (C) Dual energy x-ray absorptiometry analysis of caudal vertebra region for bone area, bone mineral content, and bone mineral density (n = 3, P < .05).
Figure 2
Skeletal phenotype in Smn−/−SMN2 mice. (A) Radiologic assessment of 4-week-old Smn−/−SMN2 (Jackson Laboratories, Bar Harbor, ME) mice shows short tails compared with wild-type mice. A high-resolution (3x) radiogram indicates pelvic bone fractures and poorly developed caudal vertebra in spinal muscular atrophy mice. (B) Dual energy x-ray absorptiometry analyses of total animal bone area and total spine for bone area, bone mineral content, and bone mineral density in spinal muscular atrophy mice. (C) Dual energy x-ray absorptiometry analysis of caudal vertebra region for bone area, bone mineral content, and bone mineral density (n = 3, P < .05).
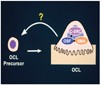
Figure 3
Osteoclast stimulatory factor and SMN protein interactions in osteoclasts. Osteoclast stimulatory factor contains a proline-rich sequence at the amino terminus, followed by an SH3 domain and ankyrin repeats. Osteoclast stimulatory factor interacts with c-Src through a proline-rich sequence. Osteoclast stimulatory factor SH3 domain binds to SMN at the exon 6 encoding region. Osteoclast stimulatory factor and SMN signaling release soluble factors that stimulate osteoclast formation and survival.
Similar articles
- Severe spinal muscular atrophy variant associated with congenital bone fractures.
Felderhoff-Mueser U, Grohmann K, Harder A, Stadelmann C, Zerres K, Bührer C, Obladen M. Felderhoff-Mueser U, et al. J Child Neurol. 2002 Sep;17(9):718-21. doi: 10.1177/088307380201700915. J Child Neurol. 2002. PMID: 12503654 - Spinal muscular atrophy with congenital fractures: postmortem analysis.
Van Toorn R, Davies J, Wilmshurst JM. Van Toorn R, et al. J Child Neurol. 2002 Sep;17(9):721-3. doi: 10.1177/088307380201700916. J Child Neurol. 2002. PMID: 12503655 - Molecular mechanisms of spinal muscular atrophy.
Sumner CJ. Sumner CJ. J Child Neurol. 2007 Aug;22(8):979-89. doi: 10.1177/0883073807305787. J Child Neurol. 2007. PMID: 17761653 Review. - Reduced survival motor neuron (Smn) gene dose in mice leads to motor neuron degeneration: an animal model for spinal muscular atrophy type III.
Jablonka S, Schrank B, Kralewski M, Rossoll W, Sendtner M. Jablonka S, et al. Hum Mol Genet. 2000 Feb 12;9(3):341-6. doi: 10.1093/hmg/9.3.341. Hum Mol Genet. 2000. PMID: 10655542 - Therapeutics development for spinal muscular atrophy.
Sumner CJ. Sumner CJ. NeuroRx. 2006 Apr;3(2):235-45. doi: 10.1016/j.nurx.2006.01.010. NeuroRx. 2006. PMID: 16554261 Free PMC article. Review.
Cited by
- Osteoclast stimulation factor 1 (Ostf1) KNOCKOUT increases trabecular bone mass in mice.
Vermeren M, Lyraki R, Wani S, Airik R, Albagha O, Mort R, Hildebrandt F, Hurd T. Vermeren M, et al. Mamm Genome. 2017 Dec;28(11-12):498-514. doi: 10.1007/s00335-017-9718-3. Epub 2017 Sep 21. Mamm Genome. 2017. PMID: 28936620 Free PMC article. - Nutritional, Gastrointestinal and Endo-Metabolic Challenges in the Management of Children with Spinal Muscular Atrophy Type 1.
Corsello A, Scatigno L, Pascuzzi MC, Calcaterra V, Dilillo D, Vizzuso S, Pelizzo G, Zoia E, Mandelli A, Govoni A, Bosetti A, Francavilla R, Indrio F, Fabiano V, Zuccotti GV, Verduci E. Corsello A, et al. Nutrients. 2021 Jul 13;13(7):2400. doi: 10.3390/nu13072400. Nutrients. 2021. PMID: 34371910 Free PMC article. Review. - Candidate proteins, metabolites and transcripts in the Biomarkers for Spinal Muscular Atrophy (BforSMA) clinical study.
Finkel RS, Crawford TO, Swoboda KJ, Kaufmann P, Juhasz P, Li X, Guo Y, Li RH, Trachtenberg F, Forrest SJ, Kobayashi DT, Chen KS, Joyce CL, Plasterer T; Pilot Study of Biomarkers for Spinal Muscular Atrophy Trial Group. Finkel RS, et al. PLoS One. 2012;7(4):e35462. doi: 10.1371/journal.pone.0035462. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558154 Free PMC article. Clinical Trial. - Bone loss in survival motor neuron (Smn(-/-) SMN2) genetic mouse model of spinal muscular atrophy.
Shanmugarajan S, Tsuruga E, Swoboda KJ, Maria BL, Ries WL, Reddy SV. Shanmugarajan S, et al. J Pathol. 2009 Sep;219(1):52-60. doi: 10.1002/path.2566. J Pathol. 2009. PMID: 19434631 Free PMC article. - The contribution of mouse models to understanding the pathogenesis of spinal muscular atrophy.
Sleigh JN, Gillingwater TH, Talbot K. Sleigh JN, et al. Dis Model Mech. 2011 Jul;4(4):457-67. doi: 10.1242/dmm.007245. Dis Model Mech. 2011. PMID: 21708901 Free PMC article. Review.
References
- Hausmanowa-Petrusewicz I, Vrbová G. Spinal muscular atrophy: a delayed development hypothesis. Neuroreport. 2005;16(7):657–661. - PubMed
- Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. - PubMed
- Le TT, Pham LT, Butchbach ME, et al. SMNΔ7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14(6):845–857. - PubMed
- Lorson CL, Strasswimmer J, Yao JM, et al. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat Genet. 1998;19(1):63–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD054599-05/HD/NICHD NIH HHS/United States
- DE 12603/DE/NIDCR NIH HHS/United States
- R01 DE012603-10/DE/NIDCR NIH HHS/United States
- R01 HD054599/HD/NICHD NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01 DE012603/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical