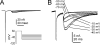The roles of sodium channels in nociception: Implications for mechanisms of pain - PubMed (original) (raw)
Review
The roles of sodium channels in nociception: Implications for mechanisms of pain
Theodore R Cummins et al. Pain. 2007 Oct.
Abstract
Understanding the role of voltage-gated sodium channels in nociception may provide important insights into pain mechanisms. Voltage-gated sodium channels are critically important for electrogenesis and nerve impulse conduction, and a target for important clinically relevant analgesics such as lidocaine. Furthermore, within the last decade studies have shown that certain sodium channel isoforms are predominantly expressed in peripheral sensory neurons associated with pain sensation, and that the expression and functional properties of voltage-gated sodium channels in peripheral sensory neurons can be dynamically regulated following axonal injury or peripheral inflammation. These data suggest that specific voltage-gated sodium channels may play crucial roles in nociception. Experiments with transgenic mice lines have clearly implicated Na(v)1.7, Na(v)1.8 and Na(v)1.9 in inflammatory, and possibly neuropathic, pain. However the most convincing and perhaps most exciting results regarding the role of voltage-gated sodium channels have come out recently from studies on human inherited disorders of nociception. Point mutations in Na(v)1.7 have been identified in patients with two distinct autosomal dominant severe chronic pain syndromes. Electrophysiological experiments indicate that these pain-associated mutations cause small yet significant changes in the gating properties of voltage-gated sodium channels that are likely to contribute substantially to the development of chronic pain. Equally exciting, recent studies indicate that recessive mutations in Na(v)1.7 that eliminate functional current can result in an apparent complete, and possibly specific, indifference to pain in humans, suggesting that isoform specific blockers could be very effective in treating pain. In this review we will examine what is known about the roles of voltage-gated sodium channels in nociception.
Figures
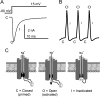
Figure 1
A, Voltage clamp recording from Nav1.7 channels showing typical voltage-gated sodium currents. The downward deflection reflects the inward movement of sodium ions in response to a depolarizing voltage pulse from a holding potential of -80 mV. The channel is closed (C) at -80 mV and when pulsed to +15 mV the channel opens (O) and rapidly inactivates (I). B, A simple action potential schematic indicating where in the action potential waveform you would expect voltage-gated sodium channels to be closed, open, or inactivated. C, Diagram indicating the proposed scheme for voltage-gated sodium channels transitions from closed to open to fast-inactivated.
Figure 2
Comparison of sodium currents recorded from a hippocampal pyramidal CA1 neuron (A) and a small diameter DRG sensory neuron (B). Sodium currents were elicited with step depolarizations from a holding potential of −100 mV to membrane voltages ranging from −80 to 40 mV. The inactivation phase of the DRG sodium currents is more complex than that of the hippocampal neuron, suggesting the presence of multiple sodium channel subtypes.
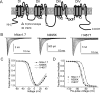
Figure 3
A, Linear diagram of a Nav1.7 voltage-gated sodium channel showing locations of single point-mutations indicated in the painful neuropathies erythromelalgia and Paroxymsal Extreme Pain Disorder (PEPD). B, Na+ current traces from the wild-type human Nav1.7 channel and from Nav1.7 channels containing an erythromelalgia mutation (N395K) and a PEPD mutation (I1461T). C, Current-voltage (IV) profile for wild-type, N395K, and I1461T Nav1.7 channels. All erythromelalgia mutations cause a hyperpolarizing shift in the voltage-dependence of activation for Nav1.7 channels while PEPD mutations thus far show no effects on activation. D, Steady-state fast inactivation profile for wild-type, N395K, and I1461T Nav1.7 channels. PEPD mutations cause a depolarizing shift in the voltage-dependence of steady-state fast inactivation while erythromelalgia mutants have little or no effect on fast inactivation properties.
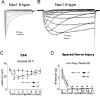
Figure 4
While the L858H mutation increases firing frequency in DRG neurons and decreases firing frequency in SCG neurons, coexpression of Nav1.8 channels rescues electrogenic properties in SCG neurons. A, Representative DRG neuron expressing wild-type Nav1.7 fires a single action potential in response to a 100 pA stimulus. B, Representative DRG neuron expressing L858H fires five action potentials in response to a 100 pA stimulus. C, Representative SCG neuron expressing WT Nav1.7 fires six action potentials in response to a 40 pA stimulus. D, Representative SCG neuron expressing L858H fires only two action potentials in response to a 100 pA stimulus. E, Action potentials recorded from representative SCG neurons transfected with wild-type Nav1.7, L858H, and L858H plus Nav1.8 channels. When Nav1.8 was coexpressed with L858H, current threshold and action potential overshoot were restored. Adapted from Rush et al., 2006a. © 2006 National Academy of Sciences, USA.
Figure 5
Comparison of Nav1.8-type TTX-R sodium currents (A) and Nav1.9-type TTX-R sodium currents (B) recorded from mouse DRG sensory neurons. C, Peripheral inflammation, induced by intraplantar injection of CFA, produced a significant reduction in hotplate latency for 7 d in wild-type and Nav1.9+/− mice, but not for Nav1.9−/− mice, indicating that Nav1.9 currents contribute to pain following peripheral inflammation. (D) Behavioral response of WT, Nav1.9 +/-, and Nav1.9-/- mice following spared nerve injury. The nerve injury produced a reduction of mechanical threshold (von Frey) that was similar in all three genotypes, indicating that Nav1.9 currents do not play a major role in neuropathic pain associated with nerve injury. C and D modified with permission from Amaya et al., 2006. © 2006 by the Society for Neuroscience.
Figure 6
TTX-sensitive resurgent currents are detected in some DRG sensory neurons. (A) Representative resurgent sodium currents recorded from a large (64 pF) sensory neuron. The voltage protocol used to elicit the currents is shown in inset. (B) The amplitudes of the traces in (A) are magnified in order to better see the resurgent currents. Adapted from Cummins et al., 2005. © 2005 Elsevier.
Similar articles
- Voltage-gated sodium channels: therapeutic targets for pain.
Dib-Hajj SD, Black JA, Waxman SG. Dib-Hajj SD, et al. Pain Med. 2009 Oct;10(7):1260-9. doi: 10.1111/j.1526-4637.2009.00719.x. Pain Med. 2009. PMID: 19818036 Review. - Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine.
Sheets PL, Heers C, Stoehr T, Cummins TR. Sheets PL, et al. J Pharmacol Exp Ther. 2008 Jul;326(1):89-99. doi: 10.1124/jpet.107.133413. Epub 2008 Mar 31. J Pharmacol Exp Ther. 2008. PMID: 18378801 - The role of voltage-gated sodium channels in neuropathic pain.
Lai J, Hunter JC, Porreca F. Lai J, et al. Curr Opin Neurobiol. 2003 Jun;13(3):291-7. doi: 10.1016/s0959-4388(03)00074-6. Curr Opin Neurobiol. 2003. PMID: 12850213 Review. - The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain.
Liu M, Wood JN. Liu M, et al. Pain Med. 2011 Jul;12 Suppl 3:S93-9. doi: 10.1111/j.1526-4637.2011.01158.x. Pain Med. 2011. PMID: 21752183 Review. - Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment.
Dib-Hajj SD, Binshtok AM, Cummins TR, Jarvis MF, Samad T, Zimmermann K. Dib-Hajj SD, et al. Brain Res Rev. 2009 Apr;60(1):65-83. doi: 10.1016/j.brainresrev.2008.12.005. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19150627 Review.
Cited by
- Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain.
Brierley SM, Hughes PA, Harrington AM, Rychkov GY, Blackshaw LA. Brierley SM, et al. Pharmaceuticals (Basel). 2010 Aug 26;3(9):2768-2798. doi: 10.3390/ph3092768. Pharmaceuticals (Basel). 2010. PMID: 27713376 Free PMC article. Review. - Multimodal General Anesthesia: Theory and Practice.
Brown EN, Pavone KJ, Naranjo M. Brown EN, et al. Anesth Analg. 2018 Nov;127(5):1246-1258. doi: 10.1213/ANE.0000000000003668. Anesth Analg. 2018. PMID: 30252709 Free PMC article. Review. - Common molecular determinants of tarantula huwentoxin-IV inhibition of Na+ channel voltage sensors in domains II and IV.
Xiao Y, Jackson JO 2nd, Liang S, Cummins TR. Xiao Y, et al. J Biol Chem. 2011 Aug 5;286(31):27301-10. doi: 10.1074/jbc.M111.246876. Epub 2011 Jun 9. J Biol Chem. 2011. PMID: 21659528 Free PMC article. - The molecular basis of pain and its clinical implications in rheumatology.
Bingham B, Ajit SK, Blake DR, Samad TA. Bingham B, et al. Nat Clin Pract Rheumatol. 2009 Jan;5(1):28-37. doi: 10.1038/ncprheum0972. Nat Clin Pract Rheumatol. 2009. PMID: 19098926 Review. - PF-06526290 can both enhance and inhibit conduction through voltage-gated sodium channels.
Wang L, Zellmer SG, Printzenhoff DM, Castle NA. Wang L, et al. Br J Pharmacol. 2018 Jul;175(14):2926-2939. doi: 10.1111/bph.14338. Epub 2018 Jun 3. Br J Pharmacol. 2018. PMID: 29791744 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
- Akopian AN, Souslova V, Sivilotti L, Wood JN. Structure and distribution of a broadly expressed atypical sodium channel. FEBS Lett. 1997;400:183–187. - PubMed
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical