Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactions - PubMed (original) (raw)
Review
. 2007;9 Suppl 2(Suppl 2):S4.
doi: 10.1186/ar2188.
Affiliations
- PMID: 17767742
- PMCID: PMC2072888
- DOI: 10.1186/ar2188
Review
Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactions
Thomas Krieg et al. Arthritis Res Ther. 2007.
Abstract
Fibrosis, characterized by excessive extracellular matrix accumulation, is a common feature of many connective tissue diseases, notably scleroderma (systemic sclerosis). Experimental studies suggest that a complex network of intercellular interactions involving endothelial cells, epithelial cells, fibroblasts and immune cells, using an array of molecular mediators, drives the pathogenic events that lead to fibrosis. Transforming growth factor-beta and endothelin-1, which are part of a cytokine hierarchy with connective tissue growth factor, are key mediators of fibrogenesis and are primarily responsible for the differentiation of fibroblasts toward a myofibroblast phenotype. The tight skin mouse (Tsk-1) model of cutaneous fibrosis suggests that numerous other genes may also be important.
Figures
Figure 1
Effects of endothelin include stimulating myofibroblast formation, leading to a concomitant increase in collagen production and fibrosis. Binding of endothelin (ET)-1 to ET-1 receptor subtype A (ETA) and ETB has different effects in different cell types. The binding of ET-1 to smooth muscle cell ETA and ETB receptors leads to vasoconstriction and mitogenesis, and activation of ETB receptors on endothelial cells promotes the release of nitric oxide and prostacyclin, and plays a minor role in endothelial dependent vasodilatation. In fibroblasts ET-1 results in the increased production of collagen and leads to fibrosis. Reproduced with permission from Galiè et al. Cardiovascular Research © Elsevier 2004 [4].
Figure 2
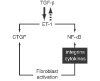
Schematic diagram of the hierarchy and interplay between ET-1, TGF-β and CTGF. CTGF, connective tissue growth factor; ET, endothelin; NF-κB, nuclear factor-κB; TGF, transforming growth factor. Reproduced from [6] by permission of the publisher (Taylor & Francis Ltd,
).
Figure 3
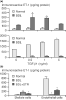
TGF-β plays a pivotal role in the induction of ET-1. (a) Stellate (top panel) and sinusoidal endothelial (bottom panel) cells were isolated from normal rat livers or those injured by bile duct ligation (BDL). Transforming growth factor (TGF)-β1 was applied at the indicated concentration in serum-free medium for 24 hours. Endothelin (ET)-1 was measured by radioimmunoassay. *P < 0.05 versus no TGF-β1 (n = 5). (b) Stellate and endothelial cells were isolated from normal rat livers, and those after BDL and those after soluble TGF-β1 receptor (STR) antagonist during BDL (BDL + STR). *P < 0.05 versus normal and *P < 0.05 versus BDL (n = 8). Reproduced with permission from Shao et al. Mol Biol Cell 2003 © The American Society for Cell Biology [9].
Figure 4
The Tsk-1 model of cutaneous fibrosis exhibits increased MAGP-2, loss of fibulin-5 and increased fibulin-2 matrix. (a) Tissue sections from tight skin (Tsk) mice (panels 1 and 3) and control (panels 2 and 4) mice were immunostained for microfibril-associated glycoprotein (MAGP)-2. MAGP-2 is indicated by arrowheads. MAGP-2 was detected at higher levels in all dermal layers (panel 1 versus panel 2). Hypodermis (H) is shown (panel 3 versus panel 4). PC, panniculus carnosus; D, dermis. (b) Control (left panel) and Tsk (right panel) mice skin sections from 6-week-old mice were stained in parallel for fibulin-5 by immunohistochemistry. Positive staining is indicated by arrows at the muscle-connective tissue (M-CT) interface. Hypodermal connective tissue (HD) and hypodermal muscle (M) are indicated. Original magnification: 400×. (c) Control (panels 1 and 3) and Tsk (panels 2 and 4) mouse skin sections from 6-week-old mice were stained in parallel for fibulin-2 by immunohistochemistry. Positive staining is indicated by arrows (M-CT interface) and in panel 4 around hair follicles (F). (a) Reproduced with permission from Lemaire et al. Arthritis Rheum 2004 © John Wiley & Sons/American College of Rheumatology [18]. (b) and (c) Reproduced with permission from [19].
Figure 5
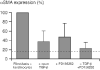
Synergistic effects of TGF-β and ET-1 in fibroblast activation. Signal intensities from Western blots of fibroblasts isolated from co-cultures of HaCaT keratinocytes and fibroblasts after 7 days in Dulbecco's modified Eagle medium/0.5% foetal calf serum. α-Smooth muscle actin (α-SMA) expression increased in co-cultures (see lane 1) as compared with fibroblasts alone (compare dotted line). Addition of an anti-transforming growth factor (TGF)-β antibody or the endothelin (ET)-1 inhibitor PD 156252 resulted in reduced α-SMA expression. Addition of anti-TGF-β and PD 156252 blocked α-SMA expression to almost basal levels [33].
Similar articles
- Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis.
Aden N, Nuttall A, Shiwen X, de Winter P, Leask A, Black CM, Denton CP, Abraham DJ, Stratton RJ. Aden N, et al. J Invest Dermatol. 2010 Sep;130(9):2191-200. doi: 10.1038/jid.2010.120. Epub 2010 May 6. J Invest Dermatol. 2010. PMID: 20445556 - Pathogenesis of scleroderma. Collagen.
Jimenez SA, Hitraya E, Varga J. Jimenez SA, et al. Rheum Dis Clin North Am. 1996 Nov;22(4):647-74. doi: 10.1016/s0889-857x(05)70294-5. Rheum Dis Clin North Am. 1996. PMID: 8923589 Review. - Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.
Cho N, Razipour SE, McCain ML. Cho N, et al. Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article. - Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast.
Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Gharaee-Kermani M, et al. Curr Med Chem. 2009;16(11):1400-17. doi: 10.2174/092986709787846497. Curr Med Chem. 2009. PMID: 19355895 Review. - New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma.
Abraham DJ, Eckes B, Rajkumar V, Krieg T. Abraham DJ, et al. Curr Rheumatol Rep. 2007 May;9(2):136-43. doi: 10.1007/s11926-007-0008-z. Curr Rheumatol Rep. 2007. PMID: 17502044 Review.
Cited by
- Microplasma Treatment versus Negative Pressure Therapy for Promoting Wound Healing in Diabetic Mice.
Shao PL, Liao JD, Wu SC, Chen YH, Wong TW. Shao PL, et al. Int J Mol Sci. 2021 Sep 24;22(19):10266. doi: 10.3390/ijms221910266. Int J Mol Sci. 2021. PMID: 34638608 Free PMC article. - CCN2: a bona fide target for anti-fibrotic drug intervention.
Leask A. Leask A. J Cell Commun Signal. 2011 Jun;5(2):131-3. doi: 10.1007/s12079-011-0125-3. Epub 2011 Mar 6. J Cell Commun Signal. 2011. PMID: 21484186 Free PMC article. - Succinate/NLRP3 Inflammasome Induces Synovial Fibroblast Activation: Therapeutical Effects of Clematichinenoside AR on Arthritis.
Li Y, Zheng JY, Liu JQ, Yang J, Liu Y, Wang C, Ma XN, Liu BL, Xin GZ, Liu LF. Li Y, et al. Front Immunol. 2016 Dec 7;7:532. doi: 10.3389/fimmu.2016.00532. eCollection 2016. Front Immunol. 2016. PMID: 28003810 Free PMC article. - Genome-wide meta-analysis reveals shared new loci in systemic seropositive rheumatic diseases.
Acosta-Herrera M, Kerick M, González-Serna D; Myositis Genetics Consortium; Scleroderma Genetics Consortium; Wijmenga C, Franke A, Gregersen PK, Padyukov L, Worthington J, Vyse TJ, Alarcón-Riquelme ME, Mayes MD, Martin J. Acosta-Herrera M, et al. Ann Rheum Dis. 2019 Mar;78(3):311-319. doi: 10.1136/annrheumdis-2018-214127. Epub 2018 Dec 20. Ann Rheum Dis. 2019. PMID: 30573655 Free PMC article. - 5Z-7-Oxozeanol Inhibits the Effects of TGFβ1 on Human Gingival Fibroblasts.
Kuk H, Hutchenreuther J, Murphy-Marshman H, Carter D, Leask A. Kuk H, et al. PLoS One. 2015 Apr 30;10(4):e0123689. doi: 10.1371/journal.pone.0123689. eCollection 2015. PLoS One. 2015. PMID: 25927238 Free PMC article.
References
- Denton CP, Black CM. Scleroderma: clinical and pathological advances. Best Pract Res Clin Rheumatol. 2004;18:271–290. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical