CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma - PubMed (original) (raw)
CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma
Takamasa Uekita et al. Mol Cell Biol. 2007 Nov.
Abstract
Malignant tumor cells frequently achieve resistance to anoikis, a form of apoptosis induced by detachment from the basement membrane, which results in the anchorage-independent growth of these cells. Although the involvement of Src family kinases (SFKs) in this alteration has been reported, little is known about the signaling pathways involved in the regulation of anoikis under the control of SFKs. In this study, we identified a membrane protein, CUB-domain-containing protein 1 (CDCP1), as an SFK-binding phosphoprotein associated with the anchorage independence of human lung adenocarcinoma. Using RNA interference suppression and overexpression of CDCP1 mutants in lung cancer cells, we found that tyrosine-phosphorylated CDCP1 is required to overcome anoikis in lung cancer cells. An apoptosis-related molecule, protein kinase Cdelta, was found to be phosphorylated by the CDCP1-SFK complex and was essential for anoikis resistance downstream of CDCP1. Loss of CDCP1 also inhibited the metastatic potential of the A549 cells in vivo. Our findings indicate that CDCP1 is a novel target for treating cancer-specific disorders, such as metastasis, by regulating anoikis in lung adenocarcinoma.
Figures
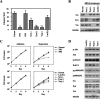
FIG. 1.
Anchorage independence of lung adenocarcinoma cells requires SFK. (A) The effect of SFKs on anchorage independence was determined by soft-agar assay. A549 cells were treated with the SFK inhibitor PP2 (10 μM) and SFK dicer RNAi [bars Src(−), Fyn(−), and Yes(−)], and controls [bars Parent, PP3, and LacZ(−)] were seeded onto each soft-agar plate (3 × 103 cells). Colonies equal to and larger than 0.5 mm in diameter were counted after 30 days. The error bars represent standard deviations, and the asterisk indicates statistically significant differences (P < 0.01) between the parent and PP2 treatment cells, while the double asterisk indicates statistically significant differences (P < 0.01) between LacZ(−) and each of the SFK RNAi treatment cells. (B) A549 cells transiently transfected with c-Src, Fyn, c-Yes, or LacZ dicer RNAi (dRNAi) were incubated for 48 h in culture plates. The cells were lysed and subjected to immunoblotting with the indicated antibodies. (C) Cell growth in A549 cells was subjected to a determination of the number of cells, as described for panel A. Approximately 2 × 104 cells were seeded onto normal (Adhesion) or MPC-coated (Suspension) culture plates with medium. The growth medium was changed every 2 days. The total cell number on each plate was determined every 2 days by a Coulter particle counter z1 (Beckman). (D) SFKs did not affect the phosphorylation of Akt, Erk1/2, or p38MAPK. The lysate of suspended A549 cells transiently transfected with dicer RNAi for each of the SFKs was prepared and subjected to immunoblotting with the indicated antibodies.
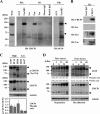
FIG. 2.
Purification of phosphotyrosine-containing 135-kDa and 70-kDa protein-forming complexes with SFKs in suspension culture. (A) Anchorage independence in a series of lung cancer cell lines was examined by soft-agar assay (top). The large number of colonies formed in the lung cancer cell lines A549, PC14, and H520 (High) and the small number of colonies formed in the H322 and H157 cell lines (Low) cultured for 48 h under both adhesion and suspension conditions were collected and subjected to immunoprecipitation (IP) with anti-Fyn (Fyn3) antibody and immunoblotting (IB) with antiphosphotyrosine (4G10) antibody. Phosphotyrosine-containing proteins coimmunoprecipitated with Fyn at the molecular masses of 135 kDa and 70 kDa are indicated by arrowheads. The asterisk indicates phosphorylated Fyn. The expression of Fyn in each cell lysate was confirmed by immunoblotting (bottom). A, adhesion; S, suspension. The error bars represent standard deviations. (B) GST-FynSH2 protein generated by Escherichia coli was used to pull down the lysate of A549 cells cultured under adhesion or suspension conditions. The isolated samples were immunoblotted with antiphosphotyrosine (4G10) antibody. The arrowheads indicate the phosphotyrosine-containing 135-kDa and 70-kDa proteins. (C) Phosphotyrosine-containing proteins (135 kDa and 70 kDa) were purified according to the protocol described in Materials and Methods. Aliquots of the purified 135-kDa and 70-kDa phosphotyrosine-containing proteins were examined by Western blotting (WB) using antiphosphotyrosine (4G10) antibody, and the remaining samples were stained with colloidal gold total-protein stain (G). Four peptides determined by mass spectrometry (peptides 1 to 4) were identified within the sequence of CDCP1.
FIG. 3.
Identification of the 135-kDa and 70-kDa proteins as CDCP1 and its phosphorylation associated with anchorage independence. (A) The lysate of A549 cells was subjected to whole-cell lysate (WCL) or pull-down assay with GST-FynSH2 protein (PD) or immunoprecipitated with anti-c-Src, anti-Fyn, and anti-c-Yes antibodies (IP) and immunoblotted (IB) with anti-CDCP1 antibody. The same blot was rehybridized with antiphosphotyrosine (4G10) antibody. (B) The lysate of A549 cells was immunoprecipitated with anti-CDCP1 antibody (ab1377) or goat IgG as indicated. The precipitates were subjected to immunoblotting with anti-c-Src, anti-Fyn, anti-c-Yes, and anti-CDCP1 antibodies. (C) The large number of colonies formed by the lung cancer cell lines A549, PC14, and H520 (High) and the small number of colonies formed by the H322 and H157 cell lines (Low) cultured for 48 h in the suspension condition were collected and subjected to immunoblotting with anti-phospho-CDCP1 (Tyr734) and CDCP1 antibodies. This experiment was performed three times. The ratio of the phosphorylation level in each lung adenocarcinoma cell was measured as described in Materials and Methods. The error bars represent standard deviations. (D) Time course analysis of CDCP1 expression and phosphorylation with or without cell attachment. A549 cells were reseeded on normal cell culture plates and an MPC-coated plate at a density of 1.5 × 105 cells per plate with complete medium. For the preparation of the reseeding cells, 2 mM EDTA/Hanks' balanced salt solution was used to detach the cells. For each time point, cells were collected and subjected to immunoblotting with the indicated antibody. The same membrane rehybridized with antitubulin antibody confirmed the concentration of total proteins in each lysate (tubulin). The arrowheads indicate CDCP1.
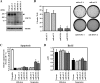
FIG. 4.
CDCP1 confers anchorage independence by inhibiting apoptosis in suspended lung adenocarcinoma. (A) CDCP1-defective A549 cell clones (miCDCP1-1 and miCDCP1-2) were generated by an miR RNAi expression vector kit (Invitrogen). miLacZ-1 and miLacZ-2 were control clones. The expression of CDCP1 in each clone (1.5 × 105 cells) cultured for 24 h in an MPC-coated plate was examined by Western blotting using CDCP1 antibody. The concentration of total protein in each clone was confirmed by the same membrane rehybridized with antitubulin antibody (bottom). The arrowheads indicate CDCP1. (B) Each CDCP1-defective clone and control clone was seeded onto soft-agar plates (3 × 103 cells) (right). Colonies equal to and larger than 0.5 mm in diameter were counted after 30 days. The error bars represent standard deviations, and the asterisks indicate statistically significant differences (P < 0.01) (left). (C) CDCP1-defective A549 cell clones (miCDCP1-1 and -2) and control miLacZ clones (1.0 × 104 cells) were cultured in normal and MPC-coated 96-well plates. After 24 h, the cells were lysed and apoptosis was examined using a cell death ELISA kit (Roche). The total apoptotic level of A549 cells was examined by treatment with etoposide (25 μM). The relative apoptosis levels are shown as the levels of apoptosis in each clone compared with those of parental cells. In suspension culture, miCDCP1 clones exhibited an increased level of apoptosis compared with that of miLacZ clones. The error bars represent standard deviations, and the asterisks indicate statistically significant differences (P < 0.01). (D) Cell proliferation was determined with a cell proliferation ELISA BrdU kit (Roche). Each clone (1.0 × 104 cells) was cultured on normal and MPC-coated 96-well plates. No significant change in cell proliferation was observed in the miCDCP1 or in miLacZ clones compared with parental A549 cells with or without cell attachment. The error bars represent standard deviations.
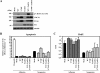
FIG. 5.
Anoikis resistance was recovered by phosphorylated CDCP1 in H322 cells with low anchorage independence. (A) H322 cells that overexpressed CDCP1 (WT), a CDCP1 mutant (Y734F), and/or Fyn kinase tagged with HA (FynHA) was incubated for 24 h in MPC-coated plates. The cells were lysed and subjected to immunoblotting with the indicated antibodies. (B) Cells, as indicated (1.0 × 104 cells), were cultured in normal and MPC-coated 96-well plates. After 24 h, the cells were lysed and apoptosis was examined using a cell death ELISA kit (Roche). The total apoptotic level of mock-infected cells was examined by treatment with etoposide (25 μM). The relative apoptosis levels are shown as the levels of apoptosis in each of the cells compared with mock-infected cells in adhesion culture. The error bars represent standard deviations, and the asterisk indicates a statistically significant difference (P < 0.05) between mock-transfected cells and other cells in suspension culture. (C) Cell proliferation was determined with a cell proliferation ELISA BrdU kit (Roche). Each of the cells (1.0 × 104 cells) was cultured on normal and MPC-coated 96-well plates. No significant change in cell proliferation was observed in each of the cells compared with mock-infected cells with or without cell attachment (BrdU). The error bars represent standard deviations.
FIG. 6.
PKCδ is a signaling molecule downstream of CDCP1 during anoikis resistance. (A) Treatment with the SFK inhibitor PP2 blocked the physical association between PKCδ and CDCP1 and at the same time suppressed phosphorylation of PKCδ at Tyr311. A549 cells treated with 10 μM of PP2 and 10 μM of PP3 in suspension culture were collected and subjected to immunoprecipitation with anti-CDCP1 antibody (ab1377) and immunoblotting (IB) with the indicated antibodies. The phospho-specific antibody against PKCδ (p-PKCδ [Τyr311]) total cell lysate was used to detect the phosphorylation of PKCδ, and the expression of PKCδ was also confirmed. (B) CDCP1 mutants were expressed in COS7 cells and pulled down (PD) with GST-FynSH2 protein. The samples pulled down were immunoblotted with FLAGM2 antibody (left). CDCP1 mutants were transiently transfected in A549 cells. After 24 h, cells were collected and subjected to immunoprecipitation (IP) with anti-FLAGM2 antibody. The immunoprecipitates were subjected to immunoblotting with the indicated antibodies. Each total cell lysate was used to detect the phosphorylation and the expression of PKCδ. (C) A549 cells treated with CDCP1 stealth siRNA and control siRNA were collected and subjected to immunoblotting with the indicated antibodies. (D) The effect of PKCδ on apoptosis was determined by apoptosis assay. PKCδ stealth siRNA was transiently transfected into CDCP1-defective A549 cell clones and control miLacZ clones. After 48 h, each cell clone (1.0 × 104 cells) was reseeded onto MPC-coated 96-well plates and cultured for 24 h. The cells were lysed and examined for apoptosis using a cell death ELISA kit (Roche). The total apoptotic level of A549 cells was examined by treatment with etoposide (25 μM). The relative apoptosis levels are shown as the level of apoptosis compared with the parent cells. The error bars represent standard deviations, and the asterisks indicate statistically significant differences (P < 0.01) between the parent and each of the other cells. Expression of CDCP1 and PKCδ was determined by Western blotting with the indicated antibodies (top). (E) The effect of PKCδ activation on apoptosis was determined by apoptosis assay. A549 cells (1.0 × 104 cells) were seeded onto MPC-coated 96-well plates and treated or not with Rottlerin (5 μM). The relative apoptosis levels after culture for 24 h are shown as the level of apoptosis compared with parent cells. The error bars represent standard deviations, and the asterisk indicates a statistically significant difference (P < 0.01) between the parent and Rottlerin-treated cells. (F) The C2 domain of PKCδ with the HA tag (C2HA) was expressed in A549 cells. After 24 h, cells were collected and subjected to immunoprecipitation with anti-CDCP1 (ab1377) or anti-HA antibody. Immunoprecipitates were subjected to immunoblotting with the indicated antibodies. Total cell lysate was used to detect the expression of C2HA and the phosphorylation level of endogenous PKCδ in A549 cells. (G) The cells transiently transfected with C2HA or mock vector, as indicated (1.0 × 104 cells), were cultured in normal and MPC-coated 96-well plates. After 24 h, the cells were lysed and apoptosis was examined using a cell death ELISA kit (Roche). The relative apoptosis levels are shown as the level of apoptosis in each of the cells compared with the control mock cells in adhesion culture. The error bars represent standard deviations, and the asterisk indicates a statistically significant difference (P < 0.05) between the mock cells and each of the other cells in suspension culture.
FIG. 7.
Metastatic capacity of CDCP1-defective lung adenocarcinoma cells. (A) The effect of CDCP1 on tumor growth in nude mice was determined as described in Materials and Methods. The data represent the weights of tumors from the miCDCP1-1 clone or the miLacZ-1 clone (n = 3). The error bars indicate standard deviations. (B) The metastatic potential was evaluated from the number of metastatic cell nodules in mouse lungs after injection of tumor cells from the tail vein (n = 6). Lung tissues were fixed with 10% formaldehyde solution. Many metastatic nodules were observed in the control A549 miLacZ-1 clone, while fewer nodules were observed in the miCDCP1-1 and miCDCP1-2 clones and H322 cells. The number of mice with obvious lung metastasis and the average number of metastatic nodules per mouse for each cell clone are shown in Table 1.
Similar articles
- Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells.
Uekita T, Fujii S, Miyazawa Y, Hashiguchi A, Abe H, Sakamoto M, Sakai R. Uekita T, et al. Cancer Sci. 2013 Jul;104(7):865-70. doi: 10.1111/cas.12154. Epub 2013 Apr 19. Cancer Sci. 2013. PMID: 23510015 Free PMC article. - CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation.
Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y, Nojima Y, Sakai R. Miyazawa Y, et al. Cancer Res. 2010 Jun 15;70(12):5136-46. doi: 10.1158/0008-5472.CAN-10-0220. Epub 2010 May 25. Cancer Res. 2010. PMID: 20501830 - Anchorage-independent phosphorylation of p130(Cas) protects lung adenocarcinoma cells from anoikis.
Wei L, Yang Y, Zhang X, Yu Q. Wei L, et al. J Cell Biochem. 2002;87(4):439-49. doi: 10.1002/jcb.10322. J Cell Biochem. 2002. PMID: 12397603 - Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis.
Uekita T, Sakai R. Uekita T, et al. Cancer Sci. 2011 Nov;102(11):1943-8. doi: 10.1111/j.1349-7006.2011.02052.x. Epub 2011 Sep 6. Cancer Sci. 2011. PMID: 21812858 Review. - Signal transduction by focal adhesion kinase in cancer.
Zhao J, Guan JL. Zhao J, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):35-49. doi: 10.1007/s10555-008-9165-4. Cancer Metastasis Rev. 2009. PMID: 19169797 Review.
Cited by
- Identification of CDCP1 as a hypoxia-inducible factor 2α (HIF-2α) target gene that is associated with survival in clear cell renal cell carcinoma patients.
Emerling BM, Benes CH, Poulogiannis G, Bell EL, Courtney K, Liu H, Choo-Wing R, Bellinger G, Tsukazawa KS, Brown V, Signoretti S, Soltoff SP, Cantley LC. Emerling BM, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3483-8. doi: 10.1073/pnas.1222435110. Epub 2013 Feb 1. Proc Natl Acad Sci U S A. 2013. PMID: 23378636 Free PMC article. - Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells.
Uekita T, Fujii S, Miyazawa Y, Hashiguchi A, Abe H, Sakamoto M, Sakai R. Uekita T, et al. Cancer Sci. 2013 Jul;104(7):865-70. doi: 10.1111/cas.12154. Epub 2013 Apr 19. Cancer Sci. 2013. PMID: 23510015 Free PMC article. - EGF inhibits constitutive internalization and palmitoylation-dependent degradation of membrane-spanning procancer CDCP1 promoting its availability on the cell surface.
Adams MN, Harrington BS, He Y, Davies CM, Wallace SJ, Chetty NP, Crandon AJ, Oliveira NB, Shannon CM, Coward JI, Lumley JW, Perrin LC, Armes JE, Hooper JD. Adams MN, et al. Oncogene. 2015 Mar 12;34(11):1375-83. doi: 10.1038/onc.2014.88. Epub 2014 Mar 31. Oncogene. 2015. PMID: 24681947 - The SRC-associated protein CUB Domain-Containing Protein-1 regulates adhesion and motility.
Benes CH, Poulogiannis G, Cantley LC, Soltoff SP. Benes CH, et al. Oncogene. 2012 Feb 2;31(5):653-63. doi: 10.1038/onc.2011.262. Epub 2011 Jul 4. Oncogene. 2012. PMID: 21725358 Free PMC article. - AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer.
Murakami Y, Kusakabe D, Watari K, Kawahara A, Azuma K, Akiba J, Taniguchi M, Kuwano M, Ono M. Murakami Y, et al. Sci Rep. 2022 May 28;12(1):8983. doi: 10.1038/s41598-022-12995-8. Sci Rep. 2022. PMID: 35643725 Free PMC article.
References
- Benes, C., and S. P. Soltoff. 2001. Modulation of PKCδ tyrosine phosphorylation and activity in salivary and PC-12 cells by Src kinases. Am. J. Physiol. Cell Physiol. 280: C1498-C1510. - PubMed
- Benes, C. H., N. Wu, A. H. Elia, T. Dharia, L. C. Cantley, and S. P. Soltoff. 2005. The C2 domain of PKCδ is a phosphotyrosine binding domain. Cell 121: 271-280. - PubMed
- Brodie, C., and P. M. Blumberg. 2003. Regulation of cell apoptosis by protein kinase C δ. Apoptosis 8: 19-27. - PubMed
- Brown, T. A., T. M. Yang, T. Zaitsevskaia, Y. Xia, C. A. Dunn, R. O. Sigle, B. Kundsen, and W. G. Carter. 2004. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J. Biol. Chem. 279: 14772-14783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous