A Hox-Eya-Pax complex regulates early kidney developmental gene expression - PubMed (original) (raw)
A Hox-Eya-Pax complex regulates early kidney developmental gene expression
Ke-Qin Gong et al. Mol Cell Biol. 2007 Nov.
Abstract
During embryonic development, the anterior-posterior body axis is specified in part by the combinatorial activities of Hox genes. Given the poor DNA binding specificity of Hox proteins, their interaction with cofactors to regulate target genes is critical. However, few regulatory partners or downstream target genes have been identified. Herein, we demonstrate that Hox11 paralogous proteins form a complex with Pax2 and Eya1 to directly activate expression of Six2 and Gdnf in the metanephric mesenchyme. We have identified the binding site within the Six2 enhancer necessary for Hox11-Eya1-Pax2-mediated activation and demonstrate that this site is essential for Six2 expression in vivo. Furthermore, genetic interactions between Hox11 and Eya1 are consistent with their participation in the same pathway. Thus, anterior-posterior-patterning Hox proteins interact with Pax2 and Eya1, factors important for nephrogenic mesoderm specification, to directly regulate the activation of downstream target genes during early kidney development.
Figures
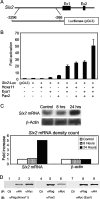
FIG. 1.
Regulation of Six2 expression by a Hox11-Eya1-Pax2 complex. (A) Schematic of the Six2-luciferase vector. A fragment from base pair 3296 to base pair 266 upstream of the Six2 ATG start site was subcloned into a luciferase expression vector. Ex1, exon 1; Ex2, exon 2. (B) Activity of the Six2 luciferase reporter plasmid in transfected MDCK cells with different combinations of Hoxa11, Pax2, and Eya1 protein expression vectors. (C) Northern blot analysis of endogenous Six2 mRNA in MDCK cells after transfection with Hoxa11, Eya1, and Pax2, normalized to β-actin. (D) Whole-cell extracts of HEK-293 cells transfected with Hoxa11-Flag, Pax2-HA, and Eya1-Myc protein expression constructs were subjected to reciprocal coimmunoprecipitations. Immunoblotting (IB) for Hoxa11 (αFlag) demonstrated coimmunoprecipitation with Pax2 (αHA) and Eya1 (αMyc) (lanes 2 and 3). Immunoblotting with Pax2 (αPax2) demonstrated coimmunoprecipitation of Hoxa11 (αFlag) and Eya1 (αMyc) (lanes 5 and 6). Immunoblotting with Eya1 (αMyc) demonstrates coimmunoprecipitation with Hoxa11 (αFlag) and Pax2 (αHA) (lanes 8 and 9). Immunopreciptations (IP) using mouse or rabbit immunoglobulin G (lanes 1, 4, and 7) were negative controls (Ctl).
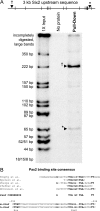
FIG. 2.
Pax2 binds regions upstream of the Six2 protein coding sequence. (A) The Pax2-PD binds two regions of the Six2 promoter in vitro. The black hatch marks indicate the HpaII sites in the 3.0-kb Six2 upstream sequence. A 5′ 222-bp region (†) and a 3′ 65-bp region (*) are pulled down only when the Pax2-PD is present. (B) Sequence analysis of the 65-bp region at bp −450 identified a putative Pax2 binding site and a putative Hox binding site based on sequence conservation to consensus sites.
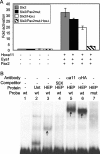
FIG. 3.
The Hox11-Eya1-Pax2 complex binds at the bp −450 region site and is necessary for Six2 expression. (A) Luciferase activities from the 3.0-kb wild-type Six2 expression construct (Six2) and constructs with the putative Pax2 binding site mutated (Six2/Pax2mut), with the Hox binding site mutated (Six2/HoxΔ), or with both the putative Pax2 and Hox sites mutated (Six2/Pax2mut-HoxΔ) were compared. All plates were cotransfected with or without Hoxa11, Pax2, and Eya1 protein expression vectors in MDCK cells. (B) An 89-bp probe (wt) containing the putative Pax2 and Hox binding sites of the Six2 promoter, incubated with nuclear extracts from HEK-293 cells transfected with Hoxa11, Eya1, and Pax2 (HEP), demonstrated retention on a nondenaturing acrylamide gel (arrow in lane 3). Probe retention was not seen in untransfected extracts (Unt; lane 2). This interaction was competed with excess (50×) unlabeled competitor (lane 4), and supershifts were observed using antibodies to Hoxa11 (αa11) or to an HA tag (αHA) on the Pax2 protein (arrows in lanes 5 and 6, respectively). The transfected extract does not show retention using a probe (mut) with the Pax2 and Hox binding sites mutated (lane 7).
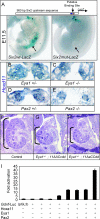
FIG. 4.
The Hox11-Eya1-Pax2 binding site is critical for kidney expression in vivo, and the Hox-Eya-Pax network synergistically activates Gdnf expression. (A) _Six2_-LacZ transient transgenic mice. The top panel shows a schematic of the 980-bp _Six2_-LacZ reporters (Hox site in red and Pax2 site in blue). (Lower left panel) E11.5 transgenic embryos carrying the wild-type _Six2_-LacZ constructs exhibit staining in the nephrogenic mesenchyme (arrow) and the branchial arches (asterisk). (Lower right panel) Transgenic mice carrying a construct with the Pax2 and Hox sites mutated retain staining in the branchial arches (asterisk; 19 of 26 embryos) but have no nephrogenic staining (arrow; 26 of 26 embryos). (B through E) No differences in the expression of Hoxd11 in the posterior intermediate mesoderm are seen in Eya1 heterozygous (B) or homozygous (C) embryos at E10.5 or in the metanephric mesenchyme of Pax2 heterozygous (D) or homozygous (E) embryos at E10.5. (F through H) Frontal hematoxylin-eosin-stained histological sections from an E14.5 control embryo (F) and embryos with three mutant Hox11 alleles plus one mutant allele of Eya1 (G and H). The brackets in panels F through H indicate relative kidney sizes. (I) Gdnf upstream sequence-driven luciferase activity in the presence of Hoxa11, Eya1, and/or Pax2 in MDCK cells.
FIG. 5.
Diagram of a proposed mechanism of Hox11 molecular function. Taken together, this work supports a model wherein Hox11 proteins form a transcriptional complex with Pax2 and Eya1 and directly activate the expression of Six2 and Gdnf during early mammalian metanephric development. Hox11P, a given Hox11 paralogous protein.
Similar articles
- Hox11 paralogous genes are essential for metanephric kidney induction.
Wellik DM, Hawkes PJ, Capecchi MR. Wellik DM, et al. Genes Dev. 2002 Jun 1;16(11):1423-32. doi: 10.1101/gad.993302. Genes Dev. 2002. PMID: 12050119 Free PMC article. - Non-homeodomain regions of Hox proteins mediate activation versus repression of Six2 via a single enhancer site in vivo.
Yallowitz AR, Gong KQ, Swinehart IT, Nelson LT, Wellik DM. Yallowitz AR, et al. Dev Biol. 2009 Nov 1;335(1):156-65. doi: 10.1016/j.ydbio.2009.08.020. Epub 2009 Aug 28. Dev Biol. 2009. PMID: 19716816 Free PMC article. - Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme.
Sajithlal G, Zou D, Silvius D, Xu PX. Sajithlal G, et al. Dev Biol. 2005 Aug 15;284(2):323-36. doi: 10.1016/j.ydbio.2005.05.029. Dev Biol. 2005. PMID: 16018995 Free PMC article. - Genetic determination of nephrogenesis: the Pax/Eya/Six gene network.
Brodbeck S, Englert C. Brodbeck S, et al. Pediatr Nephrol. 2004 Mar;19(3):249-55. doi: 10.1007/s00467-003-1374-z. Epub 2003 Dec 13. Pediatr Nephrol. 2004. PMID: 14673635 Review. - Patterning and early cell lineage decisions in the developing kidney: the role of Pax genes.
Dressler GR. Dressler GR. Pediatr Nephrol. 2011 Sep;26(9):1387-94. doi: 10.1007/s00467-010-1749-x. Epub 2011 Jan 11. Pediatr Nephrol. 2011. PMID: 21221999 Free PMC article. Review.
Cited by
- A p53-Pax2 pathway in kidney development: implications for nephrogenesis.
Saifudeen Z, Liu J, Dipp S, Yao X, Li Y, McLaughlin N, Aboudehen K, El-Dahr SS. Saifudeen Z, et al. PLoS One. 2012;7(9):e44869. doi: 10.1371/journal.pone.0044869. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984579 Free PMC article. - Kidney: polycystic kidney disease.
Paul BM, Vanden Heuvel GB. Paul BM, et al. Wiley Interdiscip Rev Dev Biol. 2014 Nov-Dec;3(6):465-87. doi: 10.1002/wdev.152. Epub 2014 Sep 3. Wiley Interdiscip Rev Dev Biol. 2014. PMID: 25186187 Free PMC article. Review. - Cell and molecular biology of kidney development.
Reidy KJ, Rosenblum ND. Reidy KJ, et al. Semin Nephrol. 2009 Jul;29(4):321-37. doi: 10.1016/j.semnephrol.2009.03.009. Semin Nephrol. 2009. PMID: 19615554 Free PMC article. Review. - Notch2 activation in the embryonic kidney depletes nephron progenitors.
Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Fujimura S, et al. J Am Soc Nephrol. 2010 May;21(5):803-10. doi: 10.1681/ASN.2009040353. Epub 2010 Mar 18. J Am Soc Nephrol. 2010. PMID: 20299358 Free PMC article. - Cellular and Molecular Mechanisms of Kidney Development: From the Embryo to the Kidney Organoid.
Khoshdel Rad N, Aghdami N, Moghadasali R. Khoshdel Rad N, et al. Front Cell Dev Biol. 2020 Mar 24;8:183. doi: 10.3389/fcell.2020.00183. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32266264 Free PMC article. Review.
References
- Benassayag, C., S. Plaza, P. Callaerts, J. Clements, Y. Romeo, W. J. Gehring, and D. L. Cribbs. 2003. Evidence for a direct functional antagonism of the selector genes proboscipedia and eyeless in Drosophila head development. Development 130: 575-586. - PubMed
- Brodbeck, S., B. Besenbeck, and C. Englert. 2004. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech. Dev. 121: 1211-1222. - PubMed
- Brodbeck, S., and C. Englert. 2004. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr. Nephrol. 19: 249-255. - PubMed
- Brophy, P. D., K. M. Lang, and G. R. Dressler. 2003. The secreted frizzled related protein 2 (SFRP2) gene is a target of the Pax2 transcription factor. J. Biol. Chem. 278: 52401-52405. - PubMed
- Brophy, P. D., L. Ostrom, K. M. Lang, and G. R. Dressler. 2001. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747-4756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK071929/DK/NIDDK NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- T32 HD007505/HD/NICHD NIH HHS/United States
- P01-DK071929/DK/NIDDK NIH HHS/United States
- CA46592/CA/NCI NIH HHS/United States
- T32-HD007505/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases