HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates - PubMed (original) (raw)
HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates
Cyril Boyault et al. Genes Dev. 2007.
Abstract
A cellular defense mechanism counteracts the deleterious effects of misfolded protein accumulation by eliciting a stress response. The cytoplasmic deacetylase HDAC6 (histone deacetylase 6) was previously shown to be a key element in this response by coordinating the clearance of protein aggregates through aggresome formation and their autophagic degradation. Here, for the first time, we demonstrate that HDAC6 is involved in another crucial cell response to the accumulation of ubiquitinated protein aggregates, and unravel its molecular basis. Indeed, our data show that HDAC6 senses ubiquitinated cellular aggregates and consequently induces the expression of major cellular chaperones by triggering the dissociation of a repressive HDAC6/HSF1 (heat-shock factor 1)/HSP90 (heat-shock protein 90) complex and a subsequent HSF1 activation. HDAC6 therefore appears as a master regulator of the cell protective response to cytotoxic protein aggregate formation.
Figures
Figure 1.
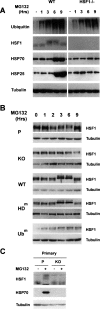
HDAC6 and its ubiquitin-binding activity are required to activate a heat-shock cell response to the proteasome inhibition. (A) 3T3 cell lines were established from mouse embryo fibroblasts isolated from parental (P) or HDAC6-deficient (KO) mice. One of the HDAC6-deficient clones was then used to establish new lines re-expressing wild-type HDAC6 (WT) or HDAC6 bearing mutations either in the two catalytic deacetylase domains (HDm) or in the ZnF-UBP ubiquitin-binding domain (Ubm). Equivalent amounts of extracts from these cells were used to compare their levels of HDAC6 expression. The blot was then successively probed with the indicated antibodies. (B) The cell lines described above, treated with 5 μg/mL MG132 for the indicated periods of time, were lysed, and extracts were prepared. Equivalent amounts of extracts were loaded, and ubiquitin, HSP25, HSP70, and tubulin were visualized on Western blots.
Figure 2.
The activation of HSF1 in response to MG132 depends on the integrity of the HDAC6 ZnF-UBP domain. (A) Embryonic MEF cells from parental (WT) and HSF1−/− cells were treated with MG132 for the indicated periods of time, and ubiquitin, HSF1, HSP70, HSP25, and tubulin expression was analyzed as above. (B) Extracts from the experiment described in Figure 1B were probed with anti-HSF1 and anti-tubulin antibodies. (C) Primary cells isolated from HDAC6-deficient (KO) and parental (P) mice were treated with MG132 for 6 h as above (+) or left untreated (−). HSF1 activation and HSP70 accumulation were monitored as shown.
Figure 3.
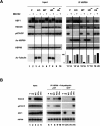
A treatment with MG132 leads to an HDAC6-dependent dissociation of the repressive HSP90–HSF1 complex. (A) Extracts from the different cell lines described in Figure 1A treated for 6 h (+) with MG132 or untreated (−) were immunoprecipitated with an anti-HSP90 antibody, and the coimmunoprecipitation of HSF1, p97/VCP, and HDAC6 was monitored. The acetylation of the immunoprecipitated HSP90 was also detected using an anti-acetylated lysin antibody. The “input” panel shows the presence of the studied proteins before immunoprecipitation in the extracts. (Right panel) The amount of HSF1 coimmunoprecipitated with HSP90 was estimated by densitometric measurement of HSF1 signals before and after MG132 treatment (shown in the HSF1 lane) and is represented as histograms. The values are expressed as a percent of HSP90-associated HSF1 before the MG132 treatment in each cell line. (B) HSP90 immunocomplexes obtained after the immunoprecipitation of HSP90, as described in A, were incubated with 10 μg of pentaubiquitin chain in the presence of 2 mM ATP/2 mM MgCl2 or not. After the elimination of the supernatant, the proteins remaining associated with the HSP90 immunocomplex were analyzed by Western blot.
Figure 4.
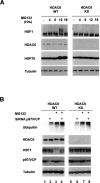
p97/VCP mediates the HDAC6-dependent HSF1 activation. (A) Human HEK cells stably expressing an anti-HDAC6 shRNA (KD) or the control cell lines (WT) (Kawaguchi et al. 2003; Kovacs et al. 2005) were treated with MG132 (5 μM, up to 18 h), and extracts were prepared after indicated periods of time. The corresponding blots were then probed with the anti-HSF1, anti-HSP70, and anti-tubulin antibodies. (B) HDAC6 knocked-down HEK (KD) and the control (WT) cell lines were treated with control siRNA or siRNA designed against p97/VCP, and cells were treated with 5 μM MG132 during 6 h as indicated. Extracts were prepared and probed with antibodies against HSF1, ubiquitin, p97/VCP, and tubulin.
Figure 5.
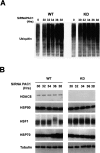
Impairment of the proteasome activity leads to an HDAC6-dependent activation of HSF1. Human HEK KD (HDAC6 knockdown) and control cells were treated with an anti-PAC1 siRNA for the indicated periods of time, and the accumulation of polyubiquitinated proteins (A) and activation of HSF1 and HSP70 (B) accumulation were monitored. The level of general protein ubiquitination and HSP70 did not change before 30 h of transfection and was comparable with that of nontransfected cells (“0” in A and not shown).
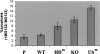
Figure 6.
HDAC6 and its ubiquitin-binding activity protect cells against the cytotoxic effects of proteasome dysfunction. The indicated cell lines (as in Fig. 1) were left untreated or were treated with 2 μM MG132 for 16 h, and the apoptotic cells were visualized by the detection of the active caspase 3 and FACS analysis. The proportion of apoptotic cells in each population was measured and was presented as a ratio of treated over untreated cells. The histograms represent three independent experiments, and the variations around the mean values are indicated. The mean values for the percentage of apoptotic cells in the untreated population are as follows: parental cells, 0.3; wild type (WT), 0.27; HDm, 0.25; KO, 0.28; Ubm, 0.21.
Similar articles
- HDAC6-ubiquitin interaction controls the duration of HSF1 activation after heat shock.
Pernet L, Faure V, Gilquin B, Dufour-Guérin S, Khochbin S, Vourc'h C. Pernet L, et al. Mol Biol Cell. 2014 Dec 15;25(25):4187-94. doi: 10.1091/mbc.E14-06-1032. Epub 2014 Oct 8. Mol Biol Cell. 2014. PMID: 25298398 Free PMC article. - Histone deacetylase 6 regulates cytotoxic α-synuclein accumulation through induction of the heat shock response.
Du Y, Wang F, Zou J, Le W, Dong Q, Wang Z, Shen F, Yu L, Li Y. Du Y, et al. Neurobiol Aging. 2014 Oct;35(10):2316-28. doi: 10.1016/j.neurobiolaging.2014.04.029. Epub 2014 May 2. Neurobiol Aging. 2014. PMID: 24866403 - Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Bali P, et al. J Biol Chem. 2005 Jul 22;280(29):26729-34. doi: 10.1074/jbc.C500186200. Epub 2005 Jun 2. J Biol Chem. 2005. PMID: 15937340 - [Histone deacetylase 6: the key regulator of misfolded proteins].
Su M, Sun X, Liu CF. Su M, et al. Sheng Li Ke Xue Jin Zhan. 2010 Apr;41(2):112-6. Sheng Li Ke Xue Jin Zhan. 2010. PMID: 21416996 Review. Chinese. - Posttranslational modification and beyond: interplay between histone deacetylase 6 and heat-shock protein 90.
Liu P, Xiao J, Wang Y, Song X, Huang L, Ren Z, Kitazato K, Wang Y. Liu P, et al. Mol Med. 2021 Sep 16;27(1):110. doi: 10.1186/s10020-021-00375-3. Mol Med. 2021. PMID: 34530730 Free PMC article. Review.
Cited by
- Targeting p97 to Disrupt Protein Homeostasis in Cancer.
Vekaria PH, Home T, Weir S, Schoenen FJ, Rao R. Vekaria PH, et al. Front Oncol. 2016 Aug 3;6:181. doi: 10.3389/fonc.2016.00181. eCollection 2016. Front Oncol. 2016. PMID: 27536557 Free PMC article. Review. - Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis.
Klingl YE, Pakravan D, Van Den Bosch L. Klingl YE, et al. Br J Pharmacol. 2021 Mar;178(6):1353-1372. doi: 10.1111/bph.15217. Epub 2020 Aug 26. Br J Pharmacol. 2021. PMID: 32726472 Free PMC article. Review. - Histone deacetylase 6 delays motor neuron degeneration by ameliorating the autophagic flux defect in a transgenic mouse model of amyotrophic lateral sclerosis.
Chen S, Zhang XJ, Li LX, Wang Y, Zhong RJ, Le W. Chen S, et al. Neurosci Bull. 2015 Aug;31(4):459-68. doi: 10.1007/s12264-015-1539-3. Epub 2015 Jul 11. Neurosci Bull. 2015. PMID: 26164555 Free PMC article. - Inhibition of HDAC6 Attenuates Tumor Growth of Non-Small Cell Lung Cancer.
Deskin B, Yin Q, Zhuang Y, Saito S, Shan B, Lasky JA. Deskin B, et al. Transl Oncol. 2020 Feb;13(2):135-145. doi: 10.1016/j.tranon.2019.11.001. Epub 2019 Dec 19. Transl Oncol. 2020. PMID: 31865176 Free PMC article. - Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA.
Kim SH, Shanware NP, Bowler MJ, Tibbetts RS. Kim SH, et al. J Biol Chem. 2010 Oct 29;285(44):34097-105. doi: 10.1074/jbc.M110.154831. Epub 2010 Aug 18. J Biol Chem. 2010. PMID: 20720006 Free PMC article.
References
- Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Kumaraswamy S., Boyapalle S., Atadja P., Boyapalle S., Atadja P., Atadja P., et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. - PubMed
- Bennett E.J., Bence N.F., Jayakumar R., Kopito R.R., Bence N.F., Jayakumar R., Kopito R.R., Jayakumar R., Kopito R.R., Kopito R.R. Global impairment of the ubiquitin–proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell. 2005;17:351–365. - PubMed
- Bertos N.R., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Yen T.J., Khochbin S., Yang X.J., Khochbin S., Yang X.J., Yang X.J. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J. Biol. Chem. 2004;279:48246–48254. - PubMed
- Boyault C., Gilquin B., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Gilquin B., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Matthias P., Muller C.W., Khochbin S., Muller C.W., Khochbin S., Khochbin S. HDAC6–p97/VCP controlled polyubiquitin chain turnover. EMBO J. 2006;25:3357–3366. - PMC - PubMed
- Christians E., Michel E., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Michel E., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Mezger V., Rallu M., Morange M., Renard J.P., Rallu M., Morange M., Renard J.P., Morange M., Renard J.P., Renard J.P. Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of the HSP70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell. Biol. 1997;17:778–788. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases