The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin - PubMed (original) (raw)
The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin
Sushant K Kachhap et al. PLoS One. 2007.
Abstract
Cell to cell adhesion is mediated by adhesion molecules present on the cell surface. Downregulation of molecules that form the adhesion complex is a characteristic of metastatic cancer cells. Downregulation of the N-myc down regulated gene1 (NDRG1) increases prostate and breast metastasis. The exact function of NDRG1 is not known. Here by using live cell confocal microscopy and in vitro reconstitution, we report that NDRG1 is involved in recycling the adhesion molecule E-cadherin thereby stabilizing it. Evidence is provided that NDRG1 recruits on recycling endosomes in the Trans Golgi network by binding to phosphotidylinositol 4-phosphate and interacts with membrane bound Rab4aGTPase. NDRG1 specifically interacts with constitutively active Rab4aQ67L mutant protein and not with GDP-bound Rab4aS22N mutant proving NDRG1 as a novel Rab4a effector. Transferrin recycling experiments reveals NDRG1 colocalizes with transferrin during the recycling phase. NDRG1 alters the kinetics of transferrin recycling in cells. NDRG1 knockdown cells show a delay in recycling transferrin, conversely NDRG1 overexpressing cells reveal an increase in rate of transferrin recycling. This novel finding of NDRG1 as a recycling protein involved with recycling of E-cadherin will aid in understanding NDRG1 role as a metastasis suppressor protein.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
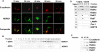
Figure 1. Presence of NDRG1 stabilizes E-cadherin in prostate cancer cells.
(A) Western blot analysis of DU-145 and LNCaP cells transfected with pSHAG1NDRG1 constructs. Control cells were transfected with pSHAG1Luc vector. Knockdown of NDRG1 downregulates E-cadherin protein levels but not other proteins of the E-cadherin complex or PCNA, actin was used as a loading control. (B) DU-145 cells transfected with pCMVNDRG1 and pCMVNDRG1flag constructs, 48 h post transfection cells were treated with cycloheximide (10 µM) for 6h and probed for E-cadherin by western blotting. E-cadherin is stabilized in cells overexpressing NDRG1 or NDRG1flag. (C) Immunohistochemical analysis of prostate cancer tissue array shows representative tumors stained for both NDRG1 and E-cadherin. Analysis of average intensity of both the proteins reveal NDRG1 and E-cadherin expression correlates significantly (n = 32, r2 = 0.8448). (D) DU145 cells transfected with NDRG1-Flag constructs were lysed with cell lysis buffer, immunoprecipitated and probed for proteins of the E-cadherin complex, none of the probed proteins of the E-cadherin complex interacts with NDRG1. (Figures A, B and D are representatives of at least three independent experiments.)
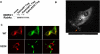
Figure 2. NDRG1 colocalizes with recycling E-cadherin and co-fractionates with recycling endosome.
(A) Immunofluorescence analysis of CWR22R cells labeled with E-cadherin antibody and chelated with EDTA. Cells were replated on calcium supplemented media and probed with primary antibody against NDRG1 and secondary antibodies against NDRG1 and E-cadherin after different time intervals. NDRG1 colocalizes with recycling E-cadherin. (B) Organelle fractions (from top to bottom) of DU-145 cells chelated with EDTA and subjected to a sucrose density gradient centrifugation were analyzed by western blotting. NDRG1 strongly localizes to a membrane organelle in cells treated with EDTA. (C) Western blotting of EDTA chelated NDRG1 positive fractions in DU-145 cells reveals NDRG1 cofractionates with E-cadherin (D) Western blotting of NDRG1 positive fractions after sucrose density gradient in HEK293 cells reveals NDRG1 co-fractionates with recycling and late endosomal markers cells after Ca2+ chelation. (All figures are representatives of at least three independent experiments.)
Figure 3. NDRG1 interacts with GTP-bound Rab4GTPase.
(A) HEK293 cells transfected with NDRG1Flag vector lysed in Tris-buffered saline, immunoprecipitated using M2-agarose and probed for different RabGTPase by western blotting. NDRG1 interacts specifically with Rab4a. Lanes 2 and 3 are immunoprecipitation from two different experiments. (B) Reciprocal immunoprecipitation using Rab4a antibody and probed with flag antibody detects a single band in NDRG1 flag transfected cell lysates. (C) Immunoprecitation of NDRG1 carried out in cell lysis buffer containing 1% TritonX100 and in Tris-buffered saline (TBS). Binding of NDRG1 to Rab4a is sensitive to TritonX100. (D) NDRG1flag purified from Drosophila S2 cells and bound to M2 agarose were incubated with purified Rab4aGST loaded with GTPγS and GDP. Bound proteins were analyzed by western blotting, NDRG1 specifically binds to Rab4aGST loaded with GTP.
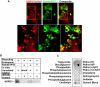
Figure 4. NDRG1 interacts with wild type and Q67L mutant of Rab4a but not with S22N mutant.
(A) Immunoprecipitation of flag-tagged wild type and mutant Rab4a reveals NDRG1 interacts specifically with wild type and GTP-bound Q67L mutant of Rab4a. (B) Confocal microscopy of NDRG1DsRed2-HEK293 stable cells reveal NDRG1 localize asymmetrically around the nucleus (N) to discrete perinuclear (PN) vesicles. NDRG1 is also seen to localize to membrane ruffles (arrow). Scale bar indicates 10µm length. (C) Wild type and GDP-bound (S22N) EGFP fusion of Rab4a transfected in NDRG1DsRed2-HEK293 stable cells reveal vesicular NDRG1 colocalizes with wild type Rab4a but not with the S22N mutant.
Figure 5. NDRG1 recruits to recycling endosome by binding to phosphatidylinositol 4-phosphate and interacts with Rab4GTPase.
(A) NDRG1DsRed2-HEK293 stable cells transfected with Rab4aQ67LEGFP mutant reveal NDRG1 interacts with Rab4aQ67LEGFP at the perinuclear region. (B) In vitro translated NDRG1 was incubated with purified recycling endosomes in the presence of recombinant GTPγS bound Rab4a and HEK293 cytosol.NDRG1 recruits on recycling endosomes independent of Rab4a or other cytosolic proteins. (C) Lipid overlay assay using purified NDRG1flag protein (1ug) shows NDRG1 binds strongly to phosphatidylinositol 4-phosphate. (All figures are representatives of at least three independent experiments.)
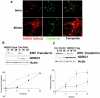
Figure 6. NDRG1 affects transferrin recycling kinetics.
(A) Live Cell confocal images of stable NDRG1DsRed2-HEK293 cells pulsed with transferrin for 5 min to load the early endosome and 60 min to load recycling/sorting vesicles reveal NDRG1DsRed2 colocalizes with recycling transferrin. The lower image panel is a still from Movie S3. (B) Transferrin recycling assay in HEK293 cells transfected with NDRG1 shRNA and control shRNA reveal a slower clearance of biotinylated transferrin from the endosomal recycling compartment (ERC) in knockdown cells. The graph below shows the percentage of recycled transferrin (see methods) in the NDRG1 knockdown (▴) as compared to control (▪) cells. The error bars represent standard deviation of 3 independent experiments. (C) Transferrin recycling assay in HEK293 cells transfected with NDRG1flag constructs and empty vector constructs reveal a faster clearance of biotinylated transferrin from the ERC in NDRG1 overexpressing cells as compared to empty control cells. The graph below shows the percentage of recycled transferrin in the NDRG1 overexpressing cells (▴) as compared to control (▪) cells.
Figure 7. Live cell confocal microscopy confirms NDRG1 involvement with recycling E-cadherin.
Live cell confocal images of stable NDRG1DsRed2-HEK293 cells transfected transiently with E-cadherinEGFP construct and plated in calcium-supplemented media after being chelated with EDTA shows NDRG1DsRed2 positive vesicles interact with recycling E-cadherinEGFP both near the perinuclear space and close to the membrane. The image is a still from Movie S4.
Similar articles
- N-myc downstream regulated gene-1/Cap43 may play an important role in malignant progression of prostate cancer, in its close association with E-cadherin.
Song Y, Oda Y, Hori M, Kuroiwa K, Ono M, Hosoi F, Basaki Y, Tokunaga S, Kuwano M, Naito S, Tsuneyoshi M. Song Y, et al. Hum Pathol. 2010 Feb;41(2):214-22. doi: 10.1016/j.humpath.2009.07.011. Epub 2009 Oct 1. Hum Pathol. 2010. PMID: 19800102 - Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy.
Fang BA, Kovačević Ž, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Fang BA, et al. Biochim Biophys Acta. 2014 Jan;1845(1):1-19. doi: 10.1016/j.bbcan.2013.11.002. Epub 2013 Nov 21. Biochim Biophys Acta. 2014. PMID: 24269900 Review. - Proteomics analysis of the interactome of N-myc downstream regulated gene 1 and its interactions with the androgen response program in prostate cancer cells.
Tu LC, Yan X, Hood L, Lin B. Tu LC, et al. Mol Cell Proteomics. 2007 Apr;6(4):575-88. doi: 10.1074/mcp.M600249-MCP200. Epub 2007 Jan 12. Mol Cell Proteomics. 2007. PMID: 17220478 - Identification and functional characterization of two missense mutations in NDRG1 associated with Charcot-Marie-Tooth disease type 4D.
Li LX, Liu GL, Liu ZJ, Lu C, Wu ZY. Li LX, et al. Hum Mutat. 2017 Nov;38(11):1569-1578. doi: 10.1002/humu.23309. Epub 2017 Aug 23. Hum Mutat. 2017. PMID: 28776325 - N-myc downstream regulated 1 gene and its place in the cellular machinery.
Kitowska A, Pawełczyk T. Kitowska A, et al. Acta Biochim Pol. 2010;57(1):15-21. Epub 2010 Mar 19. Acta Biochim Pol. 2010. PMID: 20300662 Review.
Cited by
- Charcot-Marie-Tooth disease and intracellular traffic.
Bucci C, Bakke O, Progida C. Bucci C, et al. Prog Neurobiol. 2012 Dec;99(3):191-225. doi: 10.1016/j.pneurobio.2012.03.003. Epub 2012 Mar 22. Prog Neurobiol. 2012. PMID: 22465036 Free PMC article. Review. - Lgl2 executes its function as a tumor suppressor by regulating ErbB signaling in the zebrafish epidermis.
Reischauer S, Levesque MP, Nüsslein-Volhard C, Sonawane M. Reischauer S, et al. PLoS Genet. 2009 Nov;5(11):e1000720. doi: 10.1371/journal.pgen.1000720. Epub 2009 Nov 13. PLoS Genet. 2009. PMID: 19911055 Free PMC article. - N-MYC down-regulated-like proteins regulate meristem initiation by modulating auxin transport and MAX2 expression.
Mudgil Y, Ghawana S, Jones AM. Mudgil Y, et al. PLoS One. 2013 Nov 4;8(11):e77863. doi: 10.1371/journal.pone.0077863. eCollection 2013. PLoS One. 2013. PMID: 24223735 Free PMC article. - GGA1-mediated endocytic traffic of LR11/SorLA alters APP intracellular distribution and amyloid-β production.
Herskowitz JH, Offe K, Deshpande A, Kahn RA, Levey AI, Lah JJ. Herskowitz JH, et al. Mol Biol Cell. 2012 Jul;23(14):2645-57. doi: 10.1091/mbc.E12-01-0014. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621900 Free PMC article. - Human myelin proteome and comparative analysis with mouse myelin.
Ishii A, Dutta R, Wark GM, Hwang SI, Han DK, Trapp BD, Pfeiffer SE, Bansal R. Ishii A, et al. Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14605-10. doi: 10.1073/pnas.0905936106. Epub 2009 Aug 13. Proc Natl Acad Sci U S A. 2009. PMID: 19706548 Free PMC article.
References
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. - PubMed
- Kohn EC. Invasion and metastasis: biology and clinical potential. Pharmacol Ther. 1991;52:235–244. - PubMed
- Liotta LA. Gene products which play a role in cancer invasion and metastasis. Breast Cancer Res Treat. 1988;11:113–124. - PubMed
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases