Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival - PubMed (original) (raw)
Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival
Manfred Thiel et al. PLoS One. 2007.
Abstract
Background: Sepsis patients may die either from an overwhelming systemic immune response and/or from an immunoparalysis-associated lack of anti-bacterial immune defence. We hypothesized that bacterial superantigen-activated T cells may be prevented from contribution into anti-bacterial response due to the inhibition of their effector functions by the hypoxia inducible transcription factor (HIF-1alpha) in inflamed and hypoxic areas.
Methodology/principal findings: Using the Cre-lox-P-system we generated mice with a T-cell targeted deletion of the HIF-1alpha gene and analysed them in an in vivo model of bacterial sepsis. We show that deletion of the HIF-1alpha gene leads to higher levels of pro-inflammatory cytokines, stronger anti-bacterial effects and much better survival of mice. These effects can be at least partially explained by significantly increased NF-kappaB activation in TCR activated HIF-1 alpha deficient T cells.
Conclusions/significance: T cells can be recruited to powerfully contribute to anti-bacterial response if they are relieved from inhibition by HIF-1alpha in inflamed and hypoxic areas. Our experiments uncovered the before unappreciated reserve of anti-bacterial capacity of T cells and suggest novel therapeutic anti-pathogen strategies based on targeted deletion or inhibition of HIF-1 alpha in T cells.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
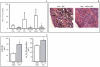
Figure 1. Increased survival and decreased bacterial sepsis-associated tissue damage of mice with T-cell targeted deletion of HIF-1α.
A:
Use of the hypoxic marker EF5 reveals that CD4+ and CD8+ T cells have been exposed to low oxygen tension (hypoxic) conditions in the peritoneum during sepsis in mice. Single cell suspensions from peritoneal lavage fluid and spleens were analyzed by flow cytometry using anti-EF5 mAb (Elk3-52 Cy5).
B:
High Efficiency of Cre recombinase-mediated deletion of HIF-1 α in T-Cells. Efficiency of deletion was calculated by quantitative real-time PCR as described. Constitutively synthesized HIF-1 α mRNA was detected in control (lck-Cre negative) but not in (lck-Cre positive) HIF1 α gene targeted mice. N = 3 per group.
C:
T cell lineage specific HIF-1 α deficient mice are more resistant to lethal sepsis after cecal ligation and puncture procedure. Mice underwent CLP and were observed for mortality. N = 13 per group. p = 0.0326, Logrank (Mantel-Cox).
D:
T cell lineage specific HIF-1 α deficient mice have less sepsis-associated liver damage as evaluated by levels of ALT transaminase activity in serum. Serum samples obtained from mice 72 hrs after CLP. *:p<0.05 vs. WT, N = 5–6 per group.
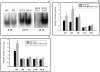
Figure 2. Decreased bacterial burden in mice with T-cell targeted deletion of HIF-1 α.
A:
T cell lineage specific HIF-1 α deficient mice have much less bacterial burden in liver and spleen 72 hrs after CLP *:p<0.05 vs. WT, means±SEM, N = 3 per group.
B:
Growth of gas-forming bacteria and tissue destruction during CLP-induced sepsis in mice with HIF-1 α_-expressing T cells, but not in mice with HIF-1_ α gene–deleted T cells. Masses of bacteria form rings around gas bubbles in spleens of mice with HIF-1 α expressing T cells. Much less bacteria could be seen in the spleen taken 72 h after CLP from mice with HIF-1 α deficiency in T cells.
C:
T-cell specific deficiency in HIF-1 α enhances effector functions of bactericidal granulocytes.
Left Panel:
Much stronger upregulation of activation marker CD11b on tissue granulocytes (CD11b+/Gr-1bright cells) isolated from HIF-1 α-deleted mice compared to HIF-1 α -expressing control mice. *:p<0.05 vs. WT, N = 3.
Right Panel:
Higher spontaneous production of hydrogen peroxide by tissue granulocytes (CD11b+/Gr-1bright cells) in HIF-1 α-deleted mice than in HIF-1 α-expressing control mice. *:p<0.05 vs. WT, N = 3.
Figure 3. HIF-1α is a negative regulator of TCR-triggered pro-inflammatory cytokine secretion in vitro and in vivo.
A:
T lymphocytes deficient in HIF-1α undergo more cell divisions as compared to wild type T cells. Splenic T cells were purified, stained using CFSE, activated for 72 hours and analyzed by flow cytometry as described. A representative dot plot of wild type CD4 T cells (A) and HIF-1α deficient T cells (B) showing both activation by CD25 expression and cell divisions. (C) Comparison of number of divisions by CD4 T cells.*: p<0.05 vs. WT, N = 3 per group.
B:
T cell specific disruption of HIF-1 α gene substantially increases pro-inflammatory cytokine secretion by ex vivo TCR-activated T cells. Spleen T cells were derived from T cell lineage specific HIF-1 α deficient mice. Cells were activated for 24 h under hypoxic conditions (1% O2). Extracellularly secreted cytokines were determined by ELISA. *:p<0.05 vs. WT, N = 5.
C:
Higher intracellular levels of IFN-γ production by inflamed peritoneum-located hypoxic HIF-1 α deficient CD8+ T cells as compared with similarly located in vivo hypoxic HIF-1 α expressing T cells after CLP. Peritoneal lavage was performed 72 hrs after CLP and 1,5×106 T cells were restimulated and stained with anti-IFN-γ mAb.
D:
Levels of proinflammatory cytokines TNF-α and IL-6 are significantly higher as compared to mice with selective disruption of HIF-1α gene in T-cells. Peritoneal fluid (TNF-α, left panel) and serum (IL-6, right panel) were withdrawn at the indicated times after CLP and cytokines were determined by ELISA. Closed circles: HIF-1α KO, open circles: WT. *:p<0.05 vs. WT, N = 4
Figure 4. Increased NF-κB m-RNA expression and activity of ex vivo activated T cells of mice with T-cell targeted deletion of HIF-1 α.
For all three panels, T-cells from spleens were isolated from age and sex matched lck Cre (−) and lck Cre (+) HIF-1α loxP mice and stimulated as described (WT-S, κΟ−S). Unstimulated cells served as controls (WT, κΟ).
A:
T cell specific disruption of HIF-1 α gene substantially increases NF-κB binding activity in ex vivo TCR-activated T cells. Nuclear extracts were prepared from harvested cells and EMSA was conducted. The experiment was repeated and representative data of two experiments are shown. All lanes contain hot binding probe for NF-κB. Specificity of EMSA was tested in the presence of 50 fold excess of either unlabeled probe (Con 1) or CRE specific probe (Con 2), respectively.
B:
T cell specific disruption of HIF-1 α gene increases NF-κB p50 and p65 binding activity in ex vivo TCR-activated T cells. NF-κB-ELISA was conducted with nuclear extracts. *:p<0.05 vs. WT, N = 4.
C:
T cell specific disruption of HIF-1 α gene increases NF-κB p50 mRNA expression in ex vivo TCR-activated T cells. RNA was prepared and subsided to quantitative RT-PCR. *:p<0.01 vs. WT. N = 4.
Similar articles
- Genetic deletion of the HIF-1α isoform I.1 in T cells enhances antibacterial immunity and improves survival in a murine peritonitis model.
Georgiev P, Belikoff BG, Hatfield S, Ohta A, Sitkovsky MV, Lukashev D. Georgiev P, et al. Eur J Immunol. 2013 Mar;43(3):655-66. doi: 10.1002/eji.201242765. Epub 2013 Jan 31. Eur J Immunol. 2013. PMID: 23208786 Free PMC article. - p66Shc is involved in promoting HIF-1alpha accumulation and cell death in hypoxic T cells.
Carraro F, Pucci A, Pellegrini M, Pelicci PG, Baldari CT, Naldini A. Carraro F, et al. J Cell Physiol. 2007 May;211(2):439-47. doi: 10.1002/jcp.20951. J Cell Physiol. 2007. PMID: 17167775 - Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes.
Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, Wenger RH, Ohta A, Sitkovsky M. Lukashev D, et al. J Immunol. 2006 Oct 15;177(8):4962-5. doi: 10.4049/jimmunol.177.8.4962. J Immunol. 2006. PMID: 17015677 - Pathophysiological response to hypoxia - from the molecular mechanisms of malady to drug discovery:inflammatory responses of hypoxia-inducible factor 1α (HIF-1α) in T cells observed in development of vascular remodeling.
Tomita S, Kihira Y, Imanishi M, Fukuhara Y, Imamura Y, Ishizawa K, Ikeda Y, Tsuchiya K, Tamaki T. Tomita S, et al. J Pharmacol Sci. 2011;115(4):433-9. doi: 10.1254/jphs.10r22fm. Epub 2011 Mar 16. J Pharmacol Sci. 2011. PMID: 21422726 Review. - Hypoxia-Inducible Factor-1α and Autoimmune Lupus, Arthritis.
Yang ZC, Liu Y. Yang ZC, et al. Inflammation. 2016 Jun;39(3):1268-73. doi: 10.1007/s10753-016-0337-z. Inflammation. 2016. PMID: 27032396 Review.
Cited by
- Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer.
Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Chouaib S, et al. Oncogene. 2017 Jan 26;36(4):439-445. doi: 10.1038/onc.2016.225. Epub 2016 Jun 27. Oncogene. 2017. PMID: 27345407 Free PMC article. Review. - Metabolic regulation of T cell differentiation and function.
Park BV, Pan F. Park BV, et al. Mol Immunol. 2015 Dec;68(2 Pt C):497-506. doi: 10.1016/j.molimm.2015.07.027. Epub 2015 Aug 12. Mol Immunol. 2015. PMID: 26277275 Free PMC article. Review. - Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells.
Cham CM, Driessens G, O'Keefe JP, Gajewski TF. Cham CM, et al. Eur J Immunol. 2008 Sep;38(9):2438-50. doi: 10.1002/eji.200838289. Eur J Immunol. 2008. PMID: 18792400 Free PMC article. - Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes.
Bellone M, Calcinotto A. Bellone M, et al. Front Oncol. 2013 Sep 11;3:231. doi: 10.3389/fonc.2013.00231. Front Oncol. 2013. PMID: 24062984 Free PMC article. Review. - Hypoxia and antitumor CD8+ T cells: An incompatible alliance?
Vuillefroy de Silly R, Dietrich PY, Walker PR. Vuillefroy de Silly R, et al. Oncoimmunology. 2016 Sep 9;5(12):e1232236. doi: 10.1080/2162402X.2016.1232236. eCollection 2016. Oncoimmunology. 2016. PMID: 28123871 Free PMC article. Review.
References
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, et al. Physiological Control of Immune Response and Inflammatory Tissue Damage by Hypoxia-Inducible Factors and Adenosine A2A Receptors. Annu Rev Immunol. 2004;22:657–82. - PubMed
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Bio. 1999;15:551–78. - PubMed
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. - PubMed
- Sitkovsky MV, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases