Behavioral and physiological consequences of sleep restriction - PubMed (original) (raw)
Review
. 2007 Aug 15;3(5):519-28.
Affiliations
- PMID: 17803017
- PMCID: PMC1978335
Review
Behavioral and physiological consequences of sleep restriction
Siobhan Banks et al. J Clin Sleep Med. 2007.
Abstract
Adequate sleep is essential for general healthy functioning. This paper reviews recent research on the effects of chronic sleep restriction on neurobehavioral and physiological functioning and discusses implications for health and lifestyle. Restricting sleep below an individual's optimal time in bed (TIB) can cause a range of neurobehavioral deficits, including lapses of attention, slowed working memory, reduced cognitive throughput, depressed mood, and perseveration of thought. Neurobehavioral deficits accumulate across days of partial sleep loss to levels equivalent to those found after 1 to 3 nights of total sleep loss. Recent experiments reveal that following days of chronic restriction of sleep duration below 7 hours per night, significant daytime cognitive dysfunction accumulates to levels comparable to that found after severe acute total sleep deprivation. Additionally, individual variability in neurobehavioral responses to sleep restriction appears to be stable, suggesting a trait-like (possibly genetic) differential vulnerability or compensatory changes in the neurobiological systems involved in cognition. A causal role for reduced sleep duration in adverse health outcomes remains unclear, but laboratory studies of healthy adults subjected to sleep restriction have found adverse effects on endocrine functions, metabolic and inflammatory responses, suggesting that sleep restriction produces physiological consequences that may be unhealthy.
Figures
Figure 1
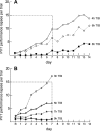
The effects of sleep restriction on NREM stage 2 sleep in Panel A; on NREM slow wave sleep (SWS) in Panel B; and on REM sleep in Panel C. Data are adapted from Van Dongen et al. Following 8 hours of time in bed on baseline nights (B1, B2, B3), sleep was restricted for 14 consecutive nights to either 4 hours of time in bed (•, n = 13 healthy adults), 6 hours of time in bed (▲, n = 13), or 8 hours of time in bed (■, n = 9). Restriction was implemented by delaying bed time and holding sleep offset time constant (07:30). Sleep restriction nights were followed by 3 nights of 10 hours of time in bed for recovery sleep (R1, R2, R3). Sleep stages were scored polysomnographically for 2 out of every 3 nights during the experiment. Panel A: During the 14 nights of restriction to 4 h of time in bed, NREM stage 2 sleep was decreased an average of more than 2 h per night relative to the 8-h control condition (p < 0.001). Stage 2 sleep was decreased approximately 1 h per night in the 6-h condition relative to the control condition (p < 0.001). Panel B: In contrast to NREM stage 2 sleep, NREM slow wave sleep (SWS) showed no significant reduction in either the 4-h or 6-h sleep restriction conditions relative to the 8-h control condition. Panel C: Relative to the 8-h control condition, REM sleep was reduced by approximately 47 minutes a night during the 14 nights of restriction to 4 h time in bed (p < 0.01), and by 24 minutes a night during the 14 nights of restriction to 6 h time in bed (p < 0.05).
Figure 2
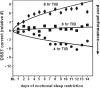
The effects of varying doses of nocturnal sleep time on lapses of attention from the psychomotor vigilance test (PVT). Panel A from Van Dongen et al. involved experimental sleep restriction of n = 36 healthy adults for 14 consecutive nights. In this experiment sleep was restricted for 14 consecutive nights. Subjects were randomized to 4 h time in bed (n = 13), 6 h time in bed (n = 13), or 8 h time in bed (n = 9). PVT performance was assessed every 2 h (9 times each day) from 07:30 to 23:30. The graph shows systematic increases in lapses of sustained attention when sleep was restricted to either 4 h (p < 0.001) or 6 h (p < 0.001) per night, but not when sleep was restricted to 8 h per night (p = 0.29). The increase in lapsing was worse in the 4-h sleep condition than in the 6-h sleep condition (p = 0.036), further supporting a dose-response relationship within and between conditions. The horizontal dotted line shows the level of lapsing found in a separate experiment when subjects had been awake continuously for 64–88 h. For example, by day 7, subjects in the 6-h sleep restriction condition averaged 54 lapses (6 lapses × 9 test times) that day, while those in the 4-h sleep condition averaged 70 lapses that day. Panel B shows comparable sleep restriction data from Belenky et al. In this study sleep was restricted for 7 consecutive nights in n = 66 healthy adults. They were randomized to 3 h time in bed (n = 13), 5 h time in bed (n = 13), 7 h time in bed (n = 13), or 9 h time in bed (n = 16). Performance was assessed 4 times each day from 09:00 to 21:00. PVT lapses increases steadily across days in the 3-h (p = 0.001) and 5-h (p = 0.001) sleep restriction conditions (PVT response speed, but not lapses, was reduced in the 7-h condition, not shown). As in Panel A, the horizontal dotted line shows the level of lapsing found in a separate experiment when subjects had been awake continuously for 64–88 h. Considering data in both Panels A and B, it is clear that restriction of nocturnal sleep time to <7 h per night in healthy adults results in systematic increases in lapses of waking attention that get progressively worse across days, in a dose-response manner.
Figure 3
Digit symbol substitution task (DSST) performance responses to varying doses of daily sleep across 14 days. Data from n = 35 subjects (8h condition n = 9, 6h condition n = 13 and 4h condition n = 13). Mean DSST per day (07:30–23:30), measured at 2-h intervals expressed relative to baseline (BL). The curves represent statistical nonlinear model-based best-fitting profiles of the DSST performance response to sleep loss. Adapted from Van Dongen et al.
Figure 4
Data from n = 35 subjects (8h condition n = 9, 6h condition n = 13 and 4h condition n = 13). Restriction of nocturnal sleep in healthy adults resulted in near-linear increases in Psychomotor Vigilance Test (PVT) lapses of attention across 14 days (coefficients of change near 1.0), but subjective ratings of sleepiness and fatigue (regardless of the psychometric scale used) showed a nonlinear coefficient below 0.5 for change over days. This meant that as objective performance continued to decline near-linearly, there were only minor further increases in the subjective ratings of sleepiness. By the end of the 14 days of sleep restriction, when performance was at its worst levels, subjects in the 4-h and 6-h sleep period conditions reported feeling only slightly sleepy. Therefore, unlike performance measures, sleepiness ratings appeared to show adaptation to chronic partial sleep deprivation. The lack of reports of intense feelings of sleepiness during chronic sleep restriction may explain why sleep restriction is widely practiced—people have the subjective impression they have adapted to it because they do not feel particularly sleepy. Adapted from Van Dongen et al.
Figure 5
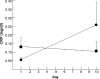
Mean (SEM) plasma high-sensitivity C-reactive protein (CRP) in n = 4 subjects undergoing 10 consecutive nights of sleep restricted to 4.2 h time in bed, and in n = 5 control subjects who had 10 consecutive nights of sleep restricted to 8.2 h time in bed (closed squares). Significance of difference in change from baseline to day 10 between groups (p = 0.08 for interaction) by mixed-models analysis of variance on log-transformed data: the change from baseline to day 10 for the 4-h sleep restriction group was significant (p = 0.05), whereas the change from baseline to day 10 in the 8-h control group was not (p = 0.72). Figure adapted from Meier-Ewert et al.
Similar articles
- The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation.
Van Dongen HP, Maislin G, Mullington JM, Dinges DF. Van Dongen HP, et al. Sleep. 2003 Mar 15;26(2):117-26. doi: 10.1093/sleep/26.2.117. Sleep. 2003. PMID: 12683469 Clinical Trial. - Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery.
Banks S, Van Dongen HP, Maislin G, Dinges DF. Banks S, et al. Sleep. 2010 Aug;33(8):1013-26. doi: 10.1093/sleep/33.8.1013. Sleep. 2010. PMID: 20815182 Free PMC article. Clinical Trial. - Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability.
Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Van Dongen HP, et al. Sleep. 2004 May 1;27(3):423-33. Sleep. 2004. PMID: 15164894 Clinical Trial. - Neurocognitive consequences of sleep deprivation.
Durmer JS, Dinges DF. Durmer JS, et al. Semin Neurol. 2005 Mar;25(1):117-29. doi: 10.1055/s-2005-867080. Semin Neurol. 2005. PMID: 15798944 Review. - Sleep deprivation and neurobehavioral dynamics.
Basner M, Rao H, Goel N, Dinges DF. Basner M, et al. Curr Opin Neurobiol. 2013 Oct;23(5):854-63. doi: 10.1016/j.conb.2013.02.008. Epub 2013 Mar 20. Curr Opin Neurobiol. 2013. PMID: 23523374 Free PMC article. Review.
Cited by
- SDIMMMER: A Proposed Clinical Approach to Optimize Cellular Physiology in Regenerative Medicine.
Lana JV, Lana JF, Melo G, Azzini GOM, Santos GS, Mosaner T, Jorge DMF, da Fonseca LF, Kruel A, Costa FR, Jeyaraman M, de Macedo AP, Santos N, Pires L, Tambeli CH. Lana JV, et al. Life (Basel). 2024 Oct 11;14(10):1287. doi: 10.3390/life14101287. Life (Basel). 2024. PMID: 39459586 Free PMC article. - Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults.
Schmidt FP, Basner M, Kröger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Münzel T. Schmidt FP, et al. Eur Heart J. 2013 Dec;34(45):3508-14a. doi: 10.1093/eurheartj/eht269. Epub 2013 Jul 2. Eur Heart J. 2013. PMID: 23821397 Free PMC article. Clinical Trial. - Objective Sleep Characteristics and Factors Associated With Sleep Duration and Waking During Pediatric Hospitalization.
Stremler R, Micsinszki S, Adams S, Parshuram C, Pullenayegum E, Weiss SK. Stremler R, et al. JAMA Netw Open. 2021 Apr 1;4(4):e213924. doi: 10.1001/jamanetworkopen.2021.3924. JAMA Netw Open. 2021. PMID: 33792731 Free PMC article. - Decreased psychomotor vigilance is a risk factor for motor vehicle crashes irrespective of subjective daytime sleepiness: the Toon Health Study.
Matsuo R, Tanigawa T, Oshima A, Tomooka K, Ikeda A, Wada H, Maruyama K, Saito I. Matsuo R, et al. J Clin Sleep Med. 2023 Feb 1;19(2):319-325. doi: 10.5664/jcsm.10328. J Clin Sleep Med. 2023. PMID: 36271594 Free PMC article. - Stimulation of the Pontine Parabrachial Nucleus Promotes Wakefulness via Extra-thalamic Forebrain Circuit Nodes.
Qiu MH, Chen MC, Fuller PM, Lu J. Qiu MH, et al. Curr Biol. 2016 Sep 12;26(17):2301-12. doi: 10.1016/j.cub.2016.07.054. Epub 2016 Aug 18. Curr Biol. 2016. PMID: 27546576 Free PMC article.
References
- Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: I. Conceptual issues. Sleep. 1989;12:1–4. - PubMed
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. - PubMed
- Dinges DF, Chugh DK. Physiologic correlates of sleep deprivation. In: Kinney JM, Tucker HN, editors. Physiology, stress, and malnutrition: Functional correlates, nutritional intervention. New York: Lippincott-Raven; 1997. pp. 1–27.
- Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28:479–96. - PubMed
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatr. 2002;59:131–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources