Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication - PubMed (original) (raw)
. 2007 Nov;81(22):12382-93.
doi: 10.1128/JVI.01543-07. Epub 2007 Sep 5.
Mark A Brockman, Ruifeng Yang, Rahma I Adam, Bin Li, Sylvie Le Gall, Charles R Rinaldo, Sharon L Craggs, Rachel L Allgaier, Karen A Power, Thomas Kuntzen, Chang-Shung Tung, Montiago X LaBute, Sandra M Mueller, Thomas Harrer, Andrew J McMichael, Philip J R Goulder, Christopher Aiken, Christian Brander, Anthony D Kelleher, Todd M Allen
Affiliations
- PMID: 17804494
- PMCID: PMC2169010
- DOI: 10.1128/JVI.01543-07
Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication
Arne Schneidewind et al. J Virol. 2007 Nov.
Abstract
Human leukocyte antigen (HLA)-B27-positive subjects are uncommon in their ability to control infection with human immunodeficiency virus type 1 (HIV-1). However, late viral escape from a narrowly directed immunodominant Gag-specific CD8(+) T-lymphocyte (CTL) response has been linked to AIDS progression in these individuals. Identifying the mechanism of the immune-mediated control may provide critical insights into HIV-1 vaccine development. Here, we illustrate that the CTL escape mutation R(264)K in the HLA-B27-restricted KK10 epitope in the capsid resulted in a significant defect in viral replication in vitro. The R(264)K variant was impaired in generating late reverse transcription products, indicating that replication was blocked at a postentry step. Notably, the R(264)K mutation was associated in vivo with the development of a rare secondary mutation, S(173)A, which restored viral replication in vitro. Furthermore, infectivity of the R(264)K variant was rescued by the addition of cyclosporine A or infection of a cyclophilin A-deficient cell line. These data demonstrate a severe functional defect imposed by the R(264)K mutation during an early step in viral replication that is likely due to the inability of this variant to replicate efficiently in the presence of normal levels of cyclophilin A. We conclude that the impact of the R(264)K substitution on capsid structure constrains viral escape and enables long-term maintenance of the dominant CTL response against B27-KK10, providing an explanation for the protective effect of HLA-B27 during HIV infection.
Figures
FIG. 1.
CTL escape in B27-KK10 is associated with multiple mutations. Viral sequences of Gag p24 in subjects expressing HLA-B27 are aligned to consensus clade B and NL4-3. Areas in gray highlight amino acid position 173 and the KK10 epitope (amino acids 263 to 272). The CypA binding domain is shown in the consensus clade B sequence (underlined), and mixed amino acids are indicated by lowercase letters. ND indicates “not determined.” cl indicates sequence derived from a single full-length clone representative of the quasispecies. Where available, viral loads are indicated. (A) Longitudinal viral sequencing in four HLA-B27+ subjects illustrates that the L268M mutation arises within the first 1 to 3 years after infection and prior to the R264K mutation. Sample time points are indicated as days postpresentation (pp). Fractions in parentheses indicate the fractions of clones representing displayed sequence. (B) Cross-sectional sequencing of 26 chronically infected B27+ subjects illustrates the propensity for viral escape through the R264K and L268M mutations. The S173A mutation is strongly associated with the R264K mutation. Numbers in parentheses indicate the number of clones sequenced for which the consensus sequence is provided.
FIG. 2.
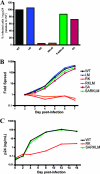
Escape mutation R264K substantially impacts viral replication in vitro. (A) CEM-GXR cells were infected with WT or variant viruses (100 μl of viral stocks), and the percentage of infected GFP-positive (GFP+) cells/ng p24 was determined at 48 h by flow cytometry. Values were normalized to WT values (black). The RK (red) and RKLM (orange) variants exhibited substantial deficiencies in infectivity, while only minor differences were observed for LM (blue), SA (purple), and SARKLM (green). Results shown are representative of three or more independent experiments. (B) CEM-GXR cells were infected (multiplicity of infection of 0.0015), and viral spread was measured over 7 days using flow cytometry to quantify GFP+ cells. The percentage of infected cells was normalized to the value at day 2 (0.15%). Again, RK (red) and RKLM (orange) exhibited deficiencies in replication, while SARKLM virus (green) replicated efficiently. Results shown are representative of three independent experiments. (C) The replicative defect of the RK variant was confirmed using primary cells. PBMC were inoculated with virus (10 ng p24), and viral spread was measured by p24 ELISA over 16 days. WT (black) and SARKLM (green) replicated similarly, while the RK variant (red) generated less p24 at all times tested. Results shown are representative of three independent experiments using different donors.
FIG. 3.
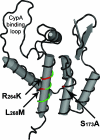
Location of KK10-associated mutations in the N-terminal domain of p24 capsid. The mutations associated with viral escape in B27-KK10 were mapped onto the structural model of the N-terminal domain of p24 capsid using Cn3D (National Center for Biotechnology Information). R264K and L268M (red) are located inside the KK10 epitope (green) on helix VII, while the S173A mutation (red) is located on helix II on the same planar surface of the folded p24 molecule as R264K and L268M.
FIG. 4.
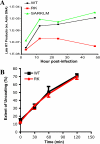
The RK variant is defective in the production of late viral reverse transcripts without affecting capsid-uncoating kinetics. (A) CEM-GXR cells were infected with VSV-g-pseudotyped WT, RK, and SARKLM viruses (400 ng p24). Cells were harvested at 3, 12, 24, and 48 h postinfection and analyzed for late reverse transcription (RT) products using real-time PCR, and values were normalized to the host cell actin gene. Late RT products were readily generated by WT (black) and SARKLM (green) within 12 h and then increased through 48 h, while the levels of reverse transcription products for RK (red) were significantly reduced at all times tested. Results are representative of two independent experiments. (B) The stabilities of WT (black) and RK (red) capsids were examined using a previously described assay (27). Purified cores were incubated in STE buffer at 37°C for the times shown. Following incubation, the samples were subjected to ultracentrifugation. The extent of disassembly was determined as the percentage of the total capsid protein in the reaction mixture present in the supernatant. In this in vitro assay, both viruses disassemble at the same rate. The results shown are mean values of duplicate determinations and are representative for two independent experiments.
FIG. 5.
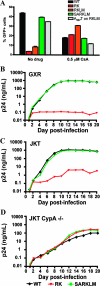
RK infectivity is restored in the absence of CypA. (A) CEM-GXR cells were infected with VSV-g-pseudotyped WT, RK, RKLM, and SARKLM viruses (400 ng p24) in the presence or absence of 0.5 μM CsA. The percentage of GFP+ cells was determined by flow cytometry at 48 h postinfection and is shown for each variant. Infectivities of WT (black) and SARKLM (green) were reduced by 2.2- and 2.5-fold, respectively, in the presence of 0.5 μM CsA, while the percentages of infected cells for RK (red) and RKLM (orange) increased by 5.7- and 3.4-fold, respectively, in the presence of drug. The introduction of A237T increased the infectivity of RKLM (gray) by 4.2-fold in the absence of CsA. In the presence of the drug, the infectivity of this strain was reduced by threefold. Results displayed are mean values for duplicate cultures and are representative of three independent experiments. To further evaluate the role of CypA, 1 million CEM-GXR (B), JKT (C), or JKT CypA−/− (D) cells were infected with 100 ng p24 equivalent of the WT (black), RK (red), or SARKLM (green). The p24 concentration in the supernatant at the indicated days postinfection was determined by ELISA. Cultures with WT and SARKLM viruses generated similar amounts of p24 in CEM-GXR and JKT cells. In the JKT CypA−/− lines, p24 production was reduced by 1 log, and peak p24 levels were delayed by 4 days. RK virus failed to increase p24 values in CEM-GXR cells. Compared to the WT and SARKLM, p24 production by RK in JKT cells was reduced by 2.5 logs, while all three viruses displayed similar p24 values for JKT Cyp−/− cells. Results shown in B, C, and D are representative of three independent experiments.
Similar articles
- Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A.
Brockman MA, Schneidewind A, Lahaie M, Schmidt A, Miura T, Desouza I, Ryvkin F, Derdeyn CA, Allen S, Hunter E, Mulenga J, Goepfert PA, Walker BD, Allen TM. Brockman MA, et al. J Virol. 2007 Nov;81(22):12608-18. doi: 10.1128/JVI.01369-07. Epub 2007 Aug 29. J Virol. 2007. PMID: 17728232 Free PMC article. - Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid.
Schneidewind A, Brockman MA, Sidney J, Wang YE, Chen H, Suscovich TJ, Li B, Adam RI, Allgaier RL, Mothé BR, Kuntzen T, Oniangue-Ndza C, Trocha A, Yu XG, Brander C, Sette A, Walker BD, Allen TM. Schneidewind A, et al. J Virol. 2008 Jun;82(11):5594-605. doi: 10.1128/JVI.02356-07. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385228 Free PMC article. - Viral evolution in HLA-B27-restricted CTL epitopes in human immunodeficiency virus type 1-infected individuals.
Setiawan LC, Gijsbers EF, van Nuenen AC, Kootstra NA. Setiawan LC, et al. J Gen Virol. 2015 Aug;96(8):2372-2380. doi: 10.1099/vir.0.000148. Epub 2015 Apr 14. J Gen Virol. 2015. PMID: 25872744 - Progression of HIV to AIDS: a protective role for HLA-B27?
den Uyl D, van der Horst-Bruinsma IE, van Agtmael M. den Uyl D, et al. AIDS Rev. 2004 Apr-Jun;6(2):89-96. AIDS Rev. 2004. PMID: 15332431 Review. - Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo?
Borrow P, Shaw GM. Borrow P, et al. Immunol Rev. 1998 Aug;164(1):37-51. doi: 10.1111/j.1600-065x.1998.tb01206.x. Immunol Rev. 1998. PMID: 9795762 Free PMC article. Review.
Cited by
- CD8+ TCR Bias and Immunodominance in HIV-1 Infection.
Kløverpris HN, McGregor R, McLaren JE, Ladell K, Harndahl M, Stryhn A, Carlson JM, Koofhethile C, Gerritsen B, Keşmir C, Chen F, Riddell L, Luzzi G, Leslie A, Walker BD, Ndung'u T, Buus S, Price DA, Goulder PJ. Kløverpris HN, et al. J Immunol. 2015 Jun 1;194(11):5329-45. doi: 10.4049/jimmunol.1400854. Epub 2015 Apr 24. J Immunol. 2015. PMID: 25911754 Free PMC article. - HIV-1 conserved-element vaccines: relationship between sequence conservation and replicative capacity.
Rolland M, Manocheewa S, Swain JV, Lanxon-Cookson EC, Kim M, Westfall DH, Larsen BB, Gilbert PB, Mullins JI. Rolland M, et al. J Virol. 2013 May;87(10):5461-7. doi: 10.1128/JVI.03033-12. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468488 Free PMC article. - Significant reductions in Gag-protease-mediated HIV-1 replication capacity during the course of the epidemic in Japan.
Nomura S, Hosoya N, Brumme ZL, Brockman MA, Kikuchi T, Koga M, Nakamura H, Koibuchi T, Fujii T, Carlson JM, Heckerman D, Kawana-Tachikawa A, Iwamoto A, Miura T. Nomura S, et al. J Virol. 2013 Feb;87(3):1465-76. doi: 10.1128/JVI.02122-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152532 Free PMC article. - Anti-APOBEC3G activity of HIV-1 Vif protein is attenuated in elite controllers.
Kikuchi T, Iwabu Y, Tada T, Kawana-Tachikawa A, Koga M, Hosoya N, Nomura S, Brumme ZL, Jessen H, Pereyra F, Trocha A, Walker BD, Iwamoto A, Tokunaga K, Miura T. Kikuchi T, et al. J Virol. 2015 May;89(9):4992-5001. doi: 10.1128/JVI.03464-14. Epub 2015 Feb 25. J Virol. 2015. PMID: 25717111 Free PMC article. - Accumulated mutations by 6 months of infection collectively render transmitted/founder HIV-1 significantly less fit.
Wang C, Liu D, Zuo T, Hora B, Cai F, Ding H, Kappes J, Ochsenbauer C, Kong W, Yu X, Bhattacharya T, Perelson AS, Gao F. Wang C, et al. J Infect. 2020 Feb;80(2):210-218. doi: 10.1016/j.jinf.2019.12.001. Epub 2019 Dec 5. J Infect. 2020. PMID: 31812703 Free PMC article.
References
- Allen, T. M., M. Altfeld, S. C. Geer, E. T. Kalife, C. Moore, K. M. O'Sullivan, I. Desouza, M. E. Feeney, R. L. Eldridge, E. L. Maier, D. E. Kaufmann, M. P. Lahaie, L. Reyor, G. Tanzi, M. N. Johnston, C. Brander, R. Draenert, J. K. Rockstroh, H. Jessen, E. S. Rosenberg, S. A. Mallal, and B. D. Walker. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 79:13239-13249. - PMC - PubMed
- Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069-7078. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI067078/AI/NIAID NIH HHS/United States
- MC_U137884177/MRC_/Medical Research Council/United Kingdom
- R01 AI050423/AI/NIAID NIH HHS/United States
- R01 AI054178/AI/NIAID NIH HHS/United States
- U01 AI052403/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials