Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points - PubMed (original) (raw)
Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points
James A Ainge et al. J Neurosci. 2007.
Abstract
The hippocampus encodes both spatial and nonspatial aspects of a rat's ongoing behavior at the single-cell level. In this study, we examined the encoding of intended destination by hippocampal (CA1) place cells during performance of a serial reversal task on a double Y-maze. On the maze, rats had to make two choices to access one of four possible goal locations, two of which contained reward. Reward locations were kept constant within blocks of 10 trials but changed between blocks, and the session of each day comprised three or more trial blocks. A disproportionate number of place fields were observed in the start box and beginning stem of the maze, relative to other locations on the maze. Forty-six percent of these place fields had different firing rates on journeys to different goal boxes. Another group of cells had place fields before the second choice point, and, of these, 44% differentiated between journeys to specific goal boxes. In a second experiment, we observed that rats with hippocampal damage made significantly more errors than control rats on the Y-maze when reward locations were reversed. Together, these results suggest that, at the start of the maze, the hippocampus encodes both current location and the intended destination of the rat, and this encoding is necessary for the flexible response to changes in reinforcement contingencies.
Figures
Figure 1.
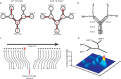
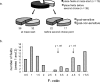
A, Schematic representation of the concatenated Y-maze. In pretraining, two of the four goal boxes contained reward in each block of 10 trials. In this example, boxes 2 and 4 are rewarded in the first 10 trials and boxes 1 and 3 in the second 10 trials. B, Representation of the binned areas used to examine the influence of goal destination on place cell firing. C, Example of the paths taken by a rat on 20 consecutive trials. The paths are ballistic and reflect little hesitation at choice points. The rat returns to the same goal on every trial until it is not rewarded and then immediately chooses another box. If that box is rewarded, it returns to this location in subsequent trials. This illustrates the ability of the rat to learn in one trial that the reward location has changed. D, Frequency distribution of place fields on the maze. Higher peaks and warmer colors indicate higher numbers of place fields.
Figure 2.
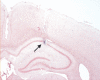
Electrode placement. The arrow indicates the mark of the electrode tip in the dorsal hippocampus.
Figure 3.
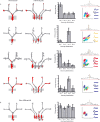
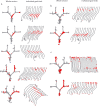
CA1 place cells in the start box encode intended destination. A, Four examples (1 on each row) of cells with place fields in the start box that fired predominantly on journeys to one of the four goal boxes. The left column shows all of the paths for a single recording session, with red dots indicating the spikes from one neuron. The shaded gray box is the place field assessed for intended trajectory. The middle left column shows the data separated into journeys to each goal box. The middle right column shows the average firing rate of the cell in the start box on journeys to each of the goal boxes. The right column shows the cluster, waveforms, and autocorrelogram of the cell. Calibration: horizontal lines, 300 μs; vertical lines, 100 μV. B, An example of a cell that had similar firing rates in the start box on journeys to all goal boxes.
Figure 4.
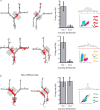
CA1 place cells before the final choice point encode intended destination. A, Two examples (1 on each row) of CA1 place cells with place fields before the final choice point that fired predominantly on trials to one of the two possible goals. The left column shows all of the trials from a single session, with the spikes from an individual neuron represented as red dots. The shaded gray box indicates the area of the maze with the place field of interest. The middle left column shows journeys to each goal box separately. The middle right column shows the average firing rate in the gray shaded area for journeys to the two possible goal boxes. The right column shows the cluster, waveforms, and autocorrelogram of the cell. Calibration: horizontal lines, 300 μs; vertical lines, 100 μV. B, An example of a cell that had similar firing rates on journeys to both goal boxes.
Figure 5.
A, Proportion of place fields in the start of the maze (areas 1 and 2) and after the start areas, but before the second choice point (top pie chart). These are broken down into the proportions of goal-sensitive and goal-nonsensitive cells in each of these two regions (bottom pie chart). B, Distribution of F ratios for goal-sensitive and goal-nonsensitive fields in the start box and first common stem of the maze (areas 1 and 2).
Figure 6.
Place field changes within a session. A, Five examples (1 on each row) of cells that developed robust place fields after several trials. The left column shows the whole session, with red dots indicating spikes fired by an individual neuron. The right column shows the individual trials to a specific goal box (the first one is on the left). B, Two examples (1 on each row) of cells that initially had robust place fields but ceased to fire at some point during the session. The left column shows the whole session, with red dots indicating spikes fired by an individual neuron. The right column shows the individual trials to a specific goal box (the first one is on the left). C, Two examples of cells (1 on each row) whose fields changed locations over maze trials.
Figure 7.
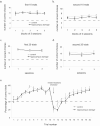
Performance on the double Y-maze task. A, The mean ± SEM number of correct choices made by the hippocampal lesion and control group in the first 10 trials of each session (reward locations constant) in the 15 postsurgery sessions (for clarity, the data are presented in 3-session blocks). The dashed line indicates the chance performance level. B, The mean ± SEM number of correct choices made in the second 10 trials of the same sessions after the reversal in reward location in the same session blocks. C, The mean ± SEM number of correct choices made in the first 20 trials of each session (reward locations constant) by the hippocampal lesion and control group in the three 40-trial testing sessions. D, The mean ± SEM number of correct choices made in the second 20 trials of the same sessions after the reversal in reward location. E, Average performance of the hippocampal lesion and control groups on each trial. Each data point is the mean ± SEM percentage of correct choices for a given trial across the 15 postsurgery testing sessions.
Figure 8.
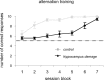
Performance on the alternation task. The mean ± SEM number of correct choices (of 10) in the 21 training sessions for both the hippocampal lesion and control groups are plotted. For clarity, the data are presented in blocks of three sessions. The dashed line is the number of correct responses expected by chance.
Similar articles
- Characteristics of CA1 place fields in a complex maze with multiple choice points.
Tanila H, Ku S, Kloosterman F, Wilson MA. Tanila H, et al. Hippocampus. 2018 Feb;28(2):81-96. doi: 10.1002/hipo.22810. Epub 2017 Nov 8. Hippocampus. 2018. PMID: 29072798 - Place cells on a maze encode routes rather than destinations.
Grieves RM, Wood ER, Dudchenko PA. Grieves RM, et al. Elife. 2016 Jun 10;5:e15986. doi: 10.7554/eLife.15986. Elife. 2016. PMID: 27282386 Free PMC article. - Relative spike timing in pairs of hippocampal neurons distinguishes the beginning and end of journeys.
Shapiro ML, Ferbinteanu J. Shapiro ML, et al. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4287-92. doi: 10.1073/pnas.0508688103. Epub 2006 Mar 3. Proc Natl Acad Sci U S A. 2006. PMID: 16537523 Free PMC article. - Hippocampal place cells encode intended destination, and not a discriminative stimulus, in a conditional T-maze task.
Ainge JA, Tamosiunaite M, Wörgötter F, Dudchenko PA. Ainge JA, et al. Hippocampus. 2012 Mar;22(3):534-43. doi: 10.1002/hipo.20919. Epub 2011 Mar 1. Hippocampus. 2012. PMID: 21365712 - Parallel processing across neural systems: implications for a multiple memory system hypothesis.
Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Mizumori SJ, et al. Neurobiol Learn Mem. 2004 Nov;82(3):278-98. doi: 10.1016/j.nlm.2004.07.007. Neurobiol Learn Mem. 2004. PMID: 15464410 Review.
Cited by
- Estimating neuronal firing density: A quantitative analysis of firing rate map algorithms.
Grieves RM. Grieves RM. PLoS Comput Biol. 2023 Dec 27;19(12):e1011763. doi: 10.1371/journal.pcbi.1011763. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38150481 Free PMC article. - Lateral entorhinal cortex is critical for novel object-context recognition.
Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. Wilson DI, et al. Hippocampus. 2013 May;23(5):352-66. doi: 10.1002/hipo.22095. Epub 2013 Feb 6. Hippocampus. 2013. PMID: 23389958 Free PMC article. - From rapid place learning to behavioral performance: a key role for the intermediate hippocampus.
Bast T, Wilson IA, Witter MP, Morris RG. Bast T, et al. PLoS Biol. 2009 Apr 21;7(4):e1000089. doi: 10.1371/journal.pbio.1000089. PLoS Biol. 2009. PMID: 19385719 Free PMC article. - Rapid, parallel path planning by propagating wavefronts of spiking neural activity.
Ponulak F, Hopfield JJ. Ponulak F, et al. Front Comput Neurosci. 2013 Jul 18;7:98. doi: 10.3389/fncom.2013.00098. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23882213 Free PMC article. - Lateralized human hippocampal activity predicts navigation based on sequence or place memory.
Iglói K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Iglói K, et al. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14466-71. doi: 10.1073/pnas.1004243107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660746 Free PMC article.
References
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167:183–195. - PubMed
- Ainge JA, van der Meer M, Langston RF, Wood ER. Exploring the role of context dependent hippocampal activity in spatial alternation behaviour. Hippocampus. 2007 in press. - PubMed
- Bahar AS, Shapiro ML. Reorganised prospective and retrospective hippocampal memory coding after switching the start and goal of a journey. Soc Neurosci Abstr. 2006;32:68–21.
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous