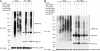Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6 - PubMed (original) (raw)
Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6
James A Olzmann et al. J Cell Biol. 2007.
Abstract
Sequestration of misfolded proteins into pericentriolar inclusions called aggresomes is a means that cells use to minimize misfolded protein-induced cytotoxicity. However, the molecular mechanism by which misfolded proteins are recruited to aggresomes remains unclear. Mutations in the E3 ligase parkin cause autosomal recessive Parkinson's disease that is devoid of Lewy bodies, which are similar to aggresomes. Here, we report that parkin cooperates with heterodimeric E2 enzyme UbcH13/Uev1a to mediate K63-linked polyubiquitination of misfolded DJ-1. K63-linked polyubiquitination of misfolded DJ-1 serves as a signal for interaction with histone deacetylase 6, an adaptor protein that binds the dynein-dynactin complex. Through this interaction, misfolded DJ-1 is linked to the dynein motor and transported to aggresomes. Furthermore, fibroblasts lacking parkin display deficits in targeting misfolded DJ-1 to aggresomes. Our findings reveal a signaling role for K63-linked polyubiquitination in dynein-mediated transport, identify parkin as a key regulator in the recruitment of misfolded DJ-1 to aggresomes, and have important implications regarding the biogenesis of Lewy bodies.
Figures
Figure 1.
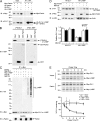
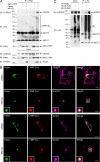
Selective interaction and ubiquitination of L166P mutant DJ-1 by parkin. (A) Lysates from transfected SH-SY5Y cells were subjected to immunoprecipitation with anti-HA antibody followed by Western blotting. endo., endogenous; IB, immunoblot; IP, immunoprecipitation. (B) [35S]-labeled DJ-1 proteins were incubated with immobilized GST or GST-parkin fusion proteins. Bound DJ-1 proteins were detected by autoradiography. (C) Transfected SH-SY5Y cells were incubated in the absence or presence of 20 μM proteasome inhibitor MG132 for 8 h before harvest. Lysates were subjected to immunoprecipitation with anti-Myc antibody followed by Western blotting. The expression of parkin in the cell lysates is confirmed by immunoblotting with anti-parkin antibody. Ubn, polyubiquitin. (D) Lysates from MG132 (20 μM)- or vehicle (0.1% DMSO)-treated SH-SY5Y cells transfected with the indicated plasmids were analyzed by Western blotting. The relative level of wild-type or mutant DJ-1 was measured by quantification of the intensity of the HA-tagged DJ-1 or L166P band and normalized to the actin level in the corresponding cell lysate. The bar graph shows the results (mean ± SEM) from at least three independent experiments. (E) The half-life of Myc-tagged wild-type and L166P mutant DJ-1 expressed in SH-SY5Y cells was analyzed by pulse chase and the protein levels quantified and plotted relative to the corresponding DJ-1 levels at 0 h.
Figure 2.
Parkin mediates K63-linked polyubiquitination of L166P mutant DJ-1 in cooperation with the UbcH13/Uev1a E2 enzyme. (A) Lysates from transfected SH-SY5Y cells were subjected to immunoprecipitation with anti-Myc antibody followed by Western blotting. (B and C) Purified L166P mutant DJ-1 was subjected to in vitro ubiquitination in the presence of E1, E2 (UbcH13/Uev1a), GST-tagged parkin, and wild-type or mutant ubiquitin as indicated. Ubiquitinated mutant DJ-1 was detected by immunoblotting with anti-DJ-1 antibody. All experiments were replicated three times with similar results.
Figure 3.
Parkin promotes K63-linked polyubiquitination of L166P mutant DJ-1 in vivo. (A and B) Transfected SH-SY5Y cells were treated with 20 μM MG132 for 8 h before harvest. Lysates (input) were subjected to immunoprecipitation with anti-Myc antibody followed by Western blotting. IgG HC, immunoglobulin heavy chain; IgG LC, immunoglobulin light chain.
Figure 4.
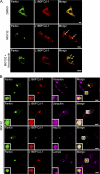
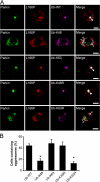
Colocalization of L166P mutant DJ-1 and parkin in perinuclear aggresomes. (A) SH-SY5Y cells coexpressing Myc-tagged parkin and HA-tagged L166P mutant DJ-1 were incubated in DME containing 0.1% DMSO, 20 μM MG132, 20 μM MG132 plus 5 μg/ml nocodazole for 16 h as indicated. In cells treated with MG132 a prominent L166P mutant DJ-1–containing aggresome is evident (arrowhead). However, cotreatment with nocodazole results in the accumulation of L166P mutant DJ-1–containing micro-aggregates (open arrowheads). (B) For further analysis of inclusions, SH-SY5Y cells coexpressing GFP-tagged parkin and Myc-tagged L166P were incubated in the presence of 20 μM MG132 for 16 h and subsequently processed for immunocytochemistry with the indicated antibodies. The colocalization between parkin and L166P mutant DJ-1 is indicated by the yellow color. The colocalization of parkin, L166P mutant DJ-1, and ubiquitin or hsp70 is indicated by the white color. Bar = 10 μm.
Figure 5.
Parkin promotes the accumulation of L166P mutant DJ-1 in aggresomes. (A–D) SH-SY5Y cells coexpressing HA-tagged wild-type DJ-1 or L166P mutant DJ-1 and Myc vector or Myc-tagged parkin were incubated in the presence and absence of 20 μM MG132 for 16 h and processed for immunofluorescence with anti-HA (red) and anti-Myc (green) antibodies. (A and B) In untreated cells HA-tagged wild-type DJ-1 and L166P mutant DJ-1 are distributed throughout the cytoplasm and colocalize with Myc-tagged parkin. (C and D) Inhibition of the proteasome coupled with overexpression of parkin resulted in the accumulation of L166P mutant DJ-1 in aggresomes (arrowheads). In cells that were not transfected with parkin L166P mutant DJ-1 mostly retained a diffuse distribution (open arrowheads). In contrast, the distribution of wild-type DJ-1 was unaffected, although these cells displayed robust parkin-containing aggresomes (arrowheads). Superimposed images revealed a colocalization (yellow) between L166P mutant DJ-1 and parkin in the perinuclear region in the presence of MG132. Bar = 10 μm. (E) Quantification shows that parkin selectively increases the targeting of L166P mutant DJ-1 to aggresomes. The asterisk indicates statistical significance (P < 0.05).
Figure 6.
Parkin increases the insolubility of L166P mutant, but not wild-type DJ-1. (A), Lysates from transfected SH-SY5Y cells were separated into detergent-soluble (S) and detergent-insoluble (I) fractions and analyzed by Western blotting. (B) The relative level of insoluble DJ-1 was measured by quantification of the intensity of the HA-tagged DJ-1 or L166P band in the detergent-insoluble fraction and normalized to the total level of HA-tagged wild-type or mutant DJ-1 in the corresponding cell lysate. The asterisk indicates a statistically significant (P < 0.05) difference compared with the corresponding control.
Figure 7.
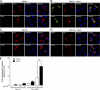
Parkin-mediated K63 polyubiquitination promotes the formation of L166P mutant DJ-1 aggresomes. (A) SH-SY5Y cells coexpressing GFP-tagged parkin, Myc-tagged L166P mutant DJ-1, and the indicated HA-tagged ubiquitin mutants were incubated in the presence of 20 μM MG132 for 16 h, fixed, and immunostained with antibodies against Myc (green) and HA (purple). Bar = 10 μm. (B) The percentage of cells containing L166P mutant DJ-1-positive aggresomes was quantified from 50–100 transfected cells and indicates that K63-linked polyubiquitination is important for the targeting of the misfolded protein to aggresomes. The asterisk indicates a statistically significant decrease (P < 0.05) in the percentage of cells containing aggresomes in cells expressing HA-tagged Ub-K48 or Ub-K63R versus the cells expressing HA-tagged Ub-WT, Ub-K63, or Ub-K48R.
Figure 8.
Parkin-mediated K63-linked polyubiquitination of L166P mutant DJ-1 promotes an association with HDAC6. (A) Lysates from transfected SH-SY5Y cells incubated in the absence or presence of MG132 were subjected to immunoprecipitation with anti-Myc antibodies followed by Western blotting. (B) SH-SY5Y cells coexpressing GFP-tagged parkin and HA-tagged L166P mutant DJ-1 were incubated in the presence and absence of MG132, immunostained as indicated, and imaged by immunofluorescence confocal microscopy. Bar = 10 μm. (C) Lysates from transfected SH-SY5Y cells were subjected to immunoprecipitation with anti-HA antibodies and analyzed by Western blotting.
Figure 9.
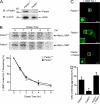
Parkin is required for proper transport of misfolded proteins to aggresomes. (A) Lysates from MEFs cultured from Parkin −/− and Parkin +/+ were separated by SDS-PAGE and analyzed by Western blotting. (B) The half-life of Myc-tagged L166P mutant DJ-1 expressed in Parkin −/− and Parkin +/+ MEFs was analyzed by pulse chase and the protein levels quantified and plotted relative to the corresponding levels at 0 h. (C) Parkin −/− and Parkin +/+ MEFs were transfected with Myc-tagged L166P mutant DJ-1 and untagged-parkin as indicated, treated with 20 μM MG132 for 16 h, and processed for immunocytochemistry with anti-Myc (green) antibodies. Bar = 10 μm. (D) The percentage of cells containing L166P mutant DJ-1 aggresomes was quantified from 50–100 transfected cells. The asterisk indicates a statistically significant difference (P < 0.05).
Figure 10.
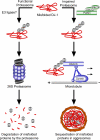
Model of parkin-dependent targeting of misfolded proteins to the aggresome. Under normal conditions misfolded proteins are recognized and conjugated with K48-linked polyubiquitin chains, resulting in efficient degradation by the 26S proteasome. However, under conditions of proteasomal impairment, parkin cooperates with Ubc13/Uev1a to mediate K63-linked polyubiquitination of misfolded proteins. This K63-linked polyubiquitin chain promotes binding of the dynein adaptor protein HDAC6, which effectively “loads” the misfolded protein onto the dynein motor complex for retrograde transport along microtubules to aggresomes.
Similar articles
- Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway.
Olzmann JA, Chin LS. Olzmann JA, et al. Autophagy. 2008 Jan;4(1):85-7. doi: 10.4161/auto.5172. Epub 2007 Oct 15. Autophagy. 2008. PMID: 17957134 Free PMC article. - Parkin Protects Against Misfolded SOD1 Toxicity by Promoting Its Aggresome Formation and Autophagic Clearance.
Yung C, Sha D, Li L, Chin LS. Yung C, et al. Mol Neurobiol. 2016 Nov;53(9):6270-6287. doi: 10.1007/s12035-015-9537-z. Epub 2015 Nov 13. Mol Neurobiol. 2016. PMID: 26563499 Free PMC article. - The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress.
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. Kawaguchi Y, et al. Cell. 2003 Dec 12;115(6):727-38. doi: 10.1016/s0092-8674(03)00939-5. Cell. 2003. PMID: 14675537 - Evidence for a common biological pathway linking three Parkinson's disease-causing genes: parkin, PINK1 and DJ-1.
van der Merwe C, Jalali Sefid Dashti Z, Christoffels A, Loos B, Bardien S. van der Merwe C, et al. Eur J Neurosci. 2015 May;41(9):1113-25. doi: 10.1111/ejn.12872. Epub 2015 Mar 11. Eur J Neurosci. 2015. PMID: 25761903 Review. - Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease.
Dodson MW, Guo M. Dodson MW, et al. Curr Opin Neurobiol. 2007 Jun;17(3):331-7. doi: 10.1016/j.conb.2007.04.010. Epub 2007 May 11. Curr Opin Neurobiol. 2007. PMID: 17499497 Review.
Cited by
- The role of autophagy in Parkinson's disease.
Zhang L, Dong Y, Xu X, Xu Z. Zhang L, et al. Neural Regen Res. 2012 Jan 15;7(2):141-5. doi: 10.3969/j.issn.1673-5374.2012.02.011. Neural Regen Res. 2012. PMID: 25767490 Free PMC article. Review. - Post-translational modification and mitochondrial function in Parkinson's disease.
Luo S, Wang D, Zhang Z. Luo S, et al. Front Mol Neurosci. 2024 Jan 11;16:1329554. doi: 10.3389/fnmol.2023.1329554. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38273938 Free PMC article. Review. - PARK2 mediates interleukin 6 and monocyte chemoattractant protein 1 production by human macrophages.
de Léséleuc L, Orlova M, Cobat A, Girard M, Huong NT, Ba NN, Thuc NV, Truman R, Spencer JS, Adams L, Thai VH, Alcais A, Schurr E. de Léséleuc L, et al. PLoS Negl Trop Dis. 2013;7(1):e2015. doi: 10.1371/journal.pntd.0002015. Epub 2013 Jan 17. PLoS Negl Trop Dis. 2013. PMID: 23350010 Free PMC article. - Ubiquitin recruiting chimera: more than just a PROTAC.
Grigoreva TA, Novikova DS, Melino G, Barlev NA, Tribulovich VG. Grigoreva TA, et al. Biol Direct. 2024 Jul 9;19(1):55. doi: 10.1186/s13062-024-00497-8. Biol Direct. 2024. PMID: 38978100 Free PMC article. Review. - Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway.
Olzmann JA, Chin LS. Olzmann JA, et al. Autophagy. 2008 Jan;4(1):85-7. doi: 10.4161/auto.5172. Epub 2007 Oct 15. Autophagy. 2008. PMID: 17957134 Free PMC article.
References
- Abou-Sleiman, P.M., D.G. Healy, N. Quinn, A.J. Lees, and N.W. Wood. 2003. The role of pathogenic DJ-1 mutations in Parkinson's disease. Ann. Neurol. 54:283–286. - PubMed
- Baulac, S., M.J. LaVoie, J. Strahle, M.G. Schlossmacher, and W. Xia. 2004. Dimerization of Parkinson's disease-causing DJ-1 and formation of high molecular weight complexes in human brain. Mol. Cell. Neurosci. 27:236–246. - PubMed
- Bence, N.F., R.M. Sampat, and R.R. Kopito. 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 292:1552–1555. - PubMed
- Bennett, E.J., N.F. Bence, R. Jayakumar, and R.R. Kopito. 2005. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell. 17:351–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 NS054597-02/NS/NINDS NIH HHS/United States
- R01 NS050650/NS/NINDS NIH HHS/United States
- AG021489/AG/NIA NIH HHS/United States
- NS050650/NS/NINDS NIH HHS/United States
- F31 NS054597-01/NS/NINDS NIH HHS/United States
- R01 AG021489/AG/NIA NIH HHS/United States
- NS047199/NS/NINDS NIH HHS/United States
- F31 NS054597/NS/NINDS NIH HHS/United States
- NS054597/NS/NINDS NIH HHS/United States
- R01 NS047199/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous