Fibrinogen induces endothelial cell permeability - PubMed (original) (raw)
Fibrinogen induces endothelial cell permeability
Neetu Tyagi et al. Mol Cell Biochem. 2008 Jan.
Abstract
Many cardiovascular and cerebrovascular disorders are accompanied by an increased blood content of fibrinogen (Fg), a high molecular weight plasma adhesion protein. Fg is a biomarker of inflammation and its degradation products have been associated with microvascular leakage. We tested the hypothesis that at pathologically high levels, Fg increases endothelial cell (EC) permeability through extracellular signal regulated kinase (ERK) signaling and by inducing F-actin formation. In cultured ECs, Fg binding to intercellular adhesion molecule-1 and to alpha(5)beta(1) integrin, caused phosphorylation of ERK. Subsequently, F-actin formation increased and coincided with formation of gaps between ECs, which corresponded with increased permeability of ECs to albumin. Our data suggest that formation of F-actin and gaps may be the mechanism for increased albumin leakage through the EC monolayer. The present study indicates that elevated un-degraded Fg may be a factor causing microvascular permeability that typically accompanies cardiovascular and cerebrovascular disorders.
Figures
Fig. 1
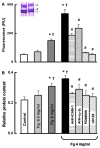
Fg-induced albumin leakage through the endothelial cell monolayer. (A) Fluorescence intensity of bovine serum albumin conjugated with Alexa Flour-555 (BSA-555) in lower chamber of Transwells; (B) Total protein content in lower chamber of Transwells relative to the total protein content in the respective upper chamber. Antibodies to ICAM-1, _α_5 and β_1 integrins, and MEK inhibitors, PD98059 or U0126, were used at concentration of 50 μM each. *P<0.05 versus control, †_P<0.05 versus lower dose of Fg. Number of experiments n = 5 for all groups. Clearly defined _α_-, _β_-, and _γ_-chains of Fg before (left) and after the cell treatment (right) shown by the Coomassie-stained SDS-PAGE analysis (reducing conditions) confirms the purity of the Fg (insert)
Fig. 2
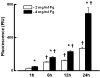
Fg leakage through the endothelial cell monolayer. Content of FITC-conjugated Fg in lower chamber of Transwells detected by fluorescence intensity measurement. *P<0.05 versus 2 mg/ml Fg, †P<0.05 versus previous time. Number of experiments n = 4
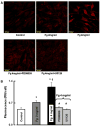
Fig. 3
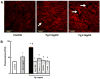
Fg-induced F-actin formation in cultured endothelial cells. (A) Examples of images of Fg-induced F-actin formation, (B) Total fluorescence intensity changes of F-actin staining in various groups of treatment. Antibodies to ICAM-1, _α_5 and β_1 integrins, and MEK inhibitors, PD98059 or U0126, were used at concentration of 50 μM each. *P<0.05 versus control. †_P<0.05 versus 2 mg/ml of Fg. #P<0.05 versus 4 mg/ml Fg alone. The arrows indicate the spaces in the endothelial cell monolayer. Each data point represents the results of duplicate determinations from four separate experiments (n = 4)
Fig. 4
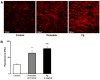
Comparison of Fg- and thrombin-induced F-actin formation in cultured endothelial cells. (A) Examples of images; (B) Fluorescence intensity of labeled F-actin associated with Fg- and thrombin-induced F-actin formation in ECs. *P<0.05 versus control. †P<0.05 versus 0.5 U/ml thrombin. Each data point represents the results of duplicate determinations from three separate experiments (n = 3)
Fig. 5
Fg-induced ERK phosphorylation in rat cardiac microvascular ECs. (A) Examples of images of Fg-induced ERK phosphorylation (images 2 and 3), and its inhibition in the presence of MEK inhibitors PD98059, and U0126 (images 4 and 5 respectively). Image 1 represents an ERK phosphorylation in cells treated with medium alone (control). (B) Fluorescence intensity per cell associated with Fg-induced ERK phosphorylation. *P<0.05 versus control, †P<0.05 versus 2 mg/ml Fg, #P<0.05 versus 4 mg/ml Fg alone. n = 4
Similar articles
- gamma-Aminbuturic acid A receptor mitigates homocysteine-induced endothelial cell permeability.
Tyagi N, Moshal KS, Tyagi SC, Lominadze D. Tyagi N, et al. Endothelium. 2007 Nov-Dec;14(6):315-23. doi: 10.1080/10623320701746164. Endothelium. 2007. PMID: 18080868 Free PMC article. - Fibrinogen-induced endothelin-1 production from endothelial cells.
Sen U, Tyagi N, Patibandla PK, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Sen U, et al. Am J Physiol Cell Physiol. 2009 Apr;296(4):C840-7. doi: 10.1152/ajpcell.00515.2008. Epub 2009 Feb 4. Am J Physiol Cell Physiol. 2009. PMID: 19193866 Free PMC article. - Fibrinogen induces alterations of endothelial cell tight junction proteins.
Patibandla PK, Tyagi N, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Patibandla PK, et al. J Cell Physiol. 2009 Oct;221(1):195-203. doi: 10.1002/jcp.21845. J Cell Physiol. 2009. PMID: 19507189 Free PMC article. - Interactions of intercellular adhesion molecule-1 with fibrinogen.
Tsakadze NL, Zhao Z, D'Souza SE. Tsakadze NL, et al. Trends Cardiovasc Med. 2002 Apr;12(3):101-8. doi: 10.1016/s1050-1738(01)00157-8. Trends Cardiovasc Med. 2002. PMID: 12007734 Review. - Mechanisms of fibrinogen-induced microvascular dysfunction during cardiovascular disease.
Lominadze D, Dean WL, Tyagi SC, Roberts AM. Lominadze D, et al. Acta Physiol (Oxf). 2010 Jan;198(1):1-13. doi: 10.1111/j.1748-1716.2009.02037.x. Epub 2009 Sep 1. Acta Physiol (Oxf). 2010. PMID: 19723026 Free PMC article. Review.
Cited by
- Cerebrovascular disorders caused by hyperfibrinogenaemia.
Muradashvili N, Tyagi R, Tyagi N, Tyagi SC, Lominadze D. Muradashvili N, et al. J Physiol. 2016 Oct 15;594(20):5941-5957. doi: 10.1113/JP272558. Epub 2016 Jun 16. J Physiol. 2016. PMID: 27121987 Free PMC article. - gamma-Aminbuturic acid A receptor mitigates homocysteine-induced endothelial cell permeability.
Tyagi N, Moshal KS, Tyagi SC, Lominadze D. Tyagi N, et al. Endothelium. 2007 Nov-Dec;14(6):315-23. doi: 10.1080/10623320701746164. Endothelium. 2007. PMID: 18080868 Free PMC article. - Control of vascular permeability by adhesion molecules.
Sarelius IH, Glading AJ. Sarelius IH, et al. Tissue Barriers. 2015 Apr 3;3(1-2):e985954. doi: 10.4161/21688370.2014.985954. eCollection 2015. Tissue Barriers. 2015. PMID: 25838987 Free PMC article. - The Deleterious Effects of Impaired Fibrinolysis on Skeletal Development Are Dependent on Fibrin(ogen), but Independent of Interlukin-6.
Cole HA, Moore-Lotridge SN, Hawley GD, Jacobson R, Yuasa M, Gewin L, Nyman JS, Flick MJ, Schoenecker JG. Cole HA, et al. Front Cardiovasc Med. 2021 Dec 6;8:768338. doi: 10.3389/fcvm.2021.768338. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34938785 Free PMC article. - Fibrinogen and Factor XIII in Venous Thrombosis and Thrombus Stability.
Wolberg AS, Sang Y. Wolberg AS, et al. Arterioscler Thromb Vasc Biol. 2022 Aug;42(8):931-941. doi: 10.1161/ATVBAHA.122.317164. Epub 2022 Jun 2. Arterioscler Thromb Vasc Biol. 2022. PMID: 35652333 Free PMC article. Review.
References
- Huxley V, Curry F. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1995;248(2 Pt 2):H264–H273. - PubMed
- Huxley V, Curry F. Effect of superfusate albumin on single capillary hydraulic conductivity. Am J Physiol Heart Circ Physiol. 1987;252(2 Pt 2):H395–H401. - PubMed
- Mehta D, Malik A. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. - PubMed
- Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. - PubMed
- Ross R. Mechanisms of disease – Atherosclerosis – An inflammatory disease. N Engl J Med. 1999;340(2):115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL074185/HL/NHLBI NIH HHS/United States
- R01 HL080394-03/HL/NHLBI NIH HHS/United States
- R01 HL080394/HL/NHLBI NIH HHS/United States
- R01 HL071010/HL/NHLBI NIH HHS/United States
- HL88012/HL/NHLBI NIH HHS/United States
- R01 NS051568/NS/NINDS NIH HHS/United States
- R01 HL088012/HL/NHLBI NIH HHS/United States
- NS51568/NS/NINDS NIH HHS/United States
- HL80394/HL/NHLBI NIH HHS/United States
- R01 HL080394-02/HL/NHLBI NIH HHS/United States
- HL71010/HL/NHLBI NIH HHS/United States
- HL74185/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous