Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex - PubMed (original) (raw)
Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex
Laura J Terry et al. J Cell Biol. 2007.
Abstract
Trafficking of nucleic acids and large proteins through nuclear pore complexes (NPCs) requires interactions with NPC proteins that harbor FG (phenylalanine-glycine) repeat domains. Specialized transport receptors that recognize cargo and bind FG domains facilitate these interactions. Whether different transport receptors utilize preferential FG domains in intact NPCs is not fully resolved. In this study, we use a large-scale deletion strategy in Saccharomyces cerevisiae to generate a new set of more minimal pore (mmp) mutants that lack specific FG domains. A comparison of messenger RNA (mRNA) export versus protein import reveals unique subsets of mmp mutants with functional defects in specific transport receptors. Thus, multiple functionally independent NPC translocation routes exist for different transport receptors. Our global analysis of the FG domain requirements in mRNA export also finds a requirement for two NPC substructures-one on the nuclear NPC face and one in the NPC central core. These results pinpoint distinct steps in the mRNA export mechanism that regulate NPC translocation efficiency.
Figures
Figure 1.
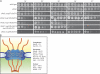
The more minimal NPC (mmp) FGΔ mutants have temperature-sensitive growth defects. (A) Wild-type, ΔNΔC, and new mmp FGΔ yeast strains were spotted onto YPD in fivefold serial dilutions and grown at the temperatures shown. (B) Schematic representation of the distribution of FG Nups within the NPC.
Figure 2.
The mmp FGΔ NPC mutants have distinct defects in Kap104 and Kap121 steady-state import. (A) Indirect immunofluorescence with an anti-Nab2 antibody in yeast mmp FGΔ strains was conducted after a 1-h shift to 37°C. Nab2 localization, indicating Kap104 import, and DAPI-staining panels are shown. (B) Localization of a Spo12-NLS-GFP reporter, which is imported by Kap121, was evaluated at 23°C and after a 1-h shift to 37°C in mmp FGΔ strains.
Figure 3.
mRNA export is inhibited in the symmetric FGΔ mutants and the mmp mutant ΔNΔ C nup57Δ GLFG. In situ hybridization with an oligo d(T) probe was conducted in the FGΔ NPC mutants after a 1-h shift to 37°C. Signal for the oligo d(T) probe indicates the subcellular distribution of poly(A)+ RNA in comparison with the nuclear signal (by coincident DAPI staining).
Figure 4.
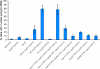
mRNA export requires the FG domains of Nup57 and nuclear face Nups. In situ hybridization with an oligo d(T) probe was conducted with the FGΔ strains indicated after a 1-h shift to 37°C. The percentage of cells showing the accumulation of poly(A)+ RNA was calculated based on fields of >100 cells in three independent trials. Deletion of the nuclear face FG domains (nup1ΔFXFG, nup2ΔFXFG, and nup60ΔFXF) is abbreviated as ΔN. Deletion of the cytoplasmic face FG domains (nup42ΔFG and nup159ΔFG) is abbreviated as ΔC. Error bars represent SEM.
Figure 5.
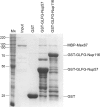
Mex67 binds the GLFG domain of Nup57. Bacterially expressed GST, GST-GLFG-NUP57, and GST-GLFG-NUP116 were each immobilized on glutathione agarose beads. Recombinant purified MBP-Mex67 was added, and the bound fraction was eluted. 10% of the input (MBP-Mex67) and the eluted fractions was resolved by SDS-PAGE and stained with Coomassie blue. Molecular mass (kilodaltons) markers are shown at the left (M r).
Figure 6.
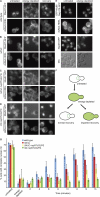
Mex67-GFP recruitment to the NE/NPC is severely inhibited in both the ΔNΔ C nup57Δ GLFG mutant and ΔN nup57Δ GLFG mutant. (A–D) Mex67-GFP localization in representative wild-type (A), ΔNΔC (B), ΔNΔC nup57ΔGLFG (C), and ΔN nup57ΔGLFG (D) cells before the assay (untreated; left), after energy depletion (middle), or after 5-6 min of recovery from energy depletion (right). For each, the coincident localization of the ER marker dsRed-HDEL is shown. (E) As controls, the localization of GFP-Nic96 and Nup170-GFP or Nup49-GFP under the same conditions was evaluated. (F) A schematic diagram of the energy depletion assay for Mex67-GFP localization is shown. (G) The kinetics of Mex67-GFP recovery to the nuclear rim over time after energy depletion was determined. For three independent experiments, >150 cells were scored for the subcellular distribution of GFP signal at each time point. Error bars represent SEM. DIC, differential interference contrast.
Similar articles
- The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo.
Zeitler B, Weis K. Zeitler B, et al. J Cell Biol. 2004 Nov 22;167(4):583-90. doi: 10.1083/jcb.200407156. J Cell Biol. 2004. PMID: 15557115 Free PMC article. - Minimal nuclear pore complexes define FG repeat domains essential for transport.
Strawn LA, Shen T, Shulga N, Goldfarb DS, Wente SR. Strawn LA, et al. Nat Cell Biol. 2004 Mar;6(3):197-206. doi: 10.1038/ncb1097. Epub 2004 Feb 22. Nat Cell Biol. 2004. PMID: 15039779 - Nucleoporin FG domains facilitate mRNP remodeling at the cytoplasmic face of the nuclear pore complex.
Adams RL, Terry LJ, Wente SR. Adams RL, et al. Genetics. 2014 Aug;197(4):1213-24. doi: 10.1534/genetics.114.164012. Epub 2014 Jun 14. Genetics. 2014. PMID: 24931410 Free PMC article. - Into the basket and beyond: the journey of mRNA through the nuclear pore complex.
Ashkenazy-Titelman A, Shav-Tal Y, Kehlenbach RH. Ashkenazy-Titelman A, et al. Biochem J. 2020 Jan 17;477(1):23-44. doi: 10.1042/BCJ20190132. Biochem J. 2020. PMID: 31913454 Review. - A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm.
Izaurralde E. Izaurralde E. Eur J Cell Biol. 2002 Nov;81(11):577-84. doi: 10.1078/0171-9335-00273. Eur J Cell Biol. 2002. PMID: 12498157 Review.
Cited by
- Nuclear distributions of NUP62 and NUP214 suggest architectural diversity and spatial patterning among nuclear pore complexes.
Kinoshita Y, Kalir T, Dottino P, Kohtz DS. Kinoshita Y, et al. PLoS One. 2012;7(4):e36137. doi: 10.1371/journal.pone.0036137. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558357 Free PMC article. - A bimodal distribution of two distinct categories of intrinsically disordered structures with separate functions in FG nucleoporins.
Yamada J, Phillips JL, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, Reza R, Acheson J, Krishnan VV, Newsam S, Gopinathan A, Lau EY, Colvin ME, Uversky VN, Rexach MF. Yamada J, et al. Mol Cell Proteomics. 2010 Oct;9(10):2205-24. doi: 10.1074/mcp.M000035-MCP201. Epub 2010 Apr 5. Mol Cell Proteomics. 2010. PMID: 20368288 Free PMC article. - Trafficking and/or division: Distinct roles of nucleoporins based on their location within the nuclear pore complex.
Hegedűsová E, Maršalová V, Kulkarni S, Paris Z. Hegedűsová E, et al. RNA Biol. 2022;19(1):650-661. doi: 10.1080/15476286.2022.2067711. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35491934 Free PMC article. - Nuclear envelope-vacuole contacts mitigate nuclear pore complex assembly stress.
Lord CL, Wente SR. Lord CL, et al. J Cell Biol. 2020 Dec 7;219(12):e202001165. doi: 10.1083/jcb.202001165. J Cell Biol. 2020. PMID: 33053148 Free PMC article. - Specific nucleoporin requirement for Smad nuclear translocation.
Chen X, Xu L. Chen X, et al. Mol Cell Biol. 2010 Aug;30(16):4022-34. doi: 10.1128/MCB.00124-10. Epub 2010 Jun 14. Mol Cell Biol. 2010. PMID: 20547758 Free PMC article.
References
- Aitchison, J.D., G. Blobel, and M.P. Rout. 1996. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 274:624–627. - PubMed
- Alcazar-Roman, A.R., E.J. Tran, S. Guo, and S.R. Wente. 2006. Inositol hexakisphosphate and Gle1 regulate Dbp5 ATPase activity in mRNA export. Nat. Cell Biol. 8:711–716. - PubMed
- Allen, N.P., L. Huang, A. Burlingame, and M. Rexach. 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276:29268–29274. - PubMed
- Bayliss, R., H.M. Kent, A.H. Corbett, and M. Stewart. 2000. a. Crystallization and initial X-ray diffraction characterization of complexes of FxFG nucleoporin repeats with nuclear transport factors. J. Struct. Biol. 131:240–247. - PubMed
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. b. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 102:99–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases