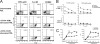4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy - PubMed (original) (raw)
4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy
Hua Zhang et al. J Immunol. 2007.
Abstract
Artificial APCs (aAPCs) genetically modified to express selective costimulatory molecules provide a reproducible, cost-effective, and convenient method for polyclonal and Ag-specific expansion of human T cells for adoptive immunotherapy. Among the variety of aAPCs that have been studied, acellular beads expressing anti-CD3/anti-CD28 efficiently expand CD4+ cells, but not CD8+ T cells. Cell-based aAPCs can effectively expand cytolytic CD8+ cells, but optimal costimulatory signals have not been defined. 4-1BB, a costimulatory molecule expressed by a minority of resting CD8+ T cells, is transiently up-regulated by all CD8+ T cells following activation. We compared expansion of human cytolytic CD8+ T cells using cell-based aAPCs providing costimulation via 4-1BB vs CD28. Whereas anti-CD3/anti-CD28 aAPCs mostly expand naive cells, anti-CD3/4-1BBL aAPCs preferentially expand memory cells, resulting in superior enrichment of Ag-reactive T cells which recognize previously primed Ags and efficient expansion of electronically sorted CD8+ populations reactive toward viral or self-Ags. Using HLA-A2-Fc fusion proteins linked to 4-1BBL aAPCs, 3-log expansion of Ag-specific CD8+ CTL was induced over 14 days, whereas similar Ag-specific CD8+ T cell expansion did not occur using HLA-A2-Fc/anti-CD28 aAPCs. Furthermore, when compared with cytolytic T cells expanded using CD28 costimulation, CTL expanded using 4-1BB costimulation mediate enhanced cytolytic capacity due, in part, to NKG2D up-regulation. These results demonstrate that 4-1BB costimulation is essential for expanding memory CD8+ T cells ex vivo and is superior to CD28 costimulation for generating Ag-specific products for adoptive cell therapy.
Figures
Figure 1
K32 cell-based aAPCs are superior to bead-based aAPCs for CD8+ expansion. A, CD8 PBL were purified and stimulated using plate-bound anti-CD3 plus anti-4-1BB (top row) or anti-CD3 plus anti-CD28 (bottom row) for the duration indicated. CD3+ CD8+ cells were gated by flow cytometry and evaluated for 4-1BB expression. Blue is binding of anti-4-1BB mAb at time 0, red is binding of anti-4-1BB mAb at time points indicated, and green is binding of anti-4-1BB mAb following preincubation with 4-1BBL-μ Ig Fc fusion protein at the time points indicated. This is a representative of three separate experiments using cells from different normal donors. B, Expansion of human CD8 T cells stimulated on with K32/anti-CD3/4-1BBL, K32/anti-CD3/anti-CD28, or anti-CD3/anti-CD28-coated magnetic beads as described in Materials and Methods. Fold cell number increase after 7 days is shown. Results are representative of more than seven different experiments, each using cells from a different normal donor. C, CD8+ cells on day 7 following expansion using anti-CD3/anti-CD28 beads demonstrate higher apoptosis compared with cells expanded using K32/anti-CD3/anti-CD28 or K32/anti-CD3/4-1BBL-based aAPCs, as evidenced by flow cytometric binding of FITC-labeled annexin V. Results are a representative of five experiments using cells from different normal donors. D, Both K32 and K32/4-1BBL aAPCs produce IL-15 and express IL-15R_α_. Intracellular IL-15 and surface IL-15R_α_ analysis was performed on K32 and K32/4-1BBL aAPCs, as well as EBV LBL serving as a negative control for IL-15 and 5838 tumor cells serving as a negative control for IL-15R_α. E_, Anti-IL-15 partially blocks CD8+ cell proliferation induced by K32/anti-CD3/4-1BBL. CD8+ T cells were coincubated with aAPC using anti-CD/4-1BBL at various ratios in the presence of anti-IL-15 mAb and isotype Ab as a control. The doses of Abs used were indicated in the figure. Proliferation was measured by [3H]thymidine incorporation during the last 16 ∼ 18 h of a 5-day culture. Results were a representative of three different experiments, using cells from different individual donors.
Figure 2
PBL costimulated with 4-1BBL, but not CD28, are enriched for memory CTL. A, CD8 PBL were unstimulated (top panel) or stimulated with K32/anti-CD3/4-1BBL (middle panel) or K32/anti-CD3/anti-CD28 (lower panel) for 7 days, then expanded cells were assayed using HLA A*0201 tetramers loaded with CMV pp65, Flu M1 peptide, and ERBB2 peptides. Anti-CD3/4-1BBL-expanded cells, but not anti-CD3/anti-CD28-expanded cells show increased frequencies of cells responding to viral recall Ags. Percentages reflect the percent of tetramer binding of CD8+ cells. B, CD8 PBL stimulated with K32/anti-CD3/4-1BBL, but not K32/anti-CD3/anti-CD28, demonstrate cytotoxicity to FluM1- and pp65-pulsed T2 targets on day 7. Results were representative of five different experiments, using cells from different individual donors. C, 4-1BB costimulation preferentially expands memory CD8+ cells but anti-CD28 costimulation preferentially expands naive CD8 cells. CD8 T cells were separated into naive (CD45RO−) and memory (CD45RO+) subsets using anti-CD45RO microbeads, then placed into a 96-well flat-bottom plate coated with anti-CD3 (1 _μ_g/ml) and anti-CD28 or anti-4-1BB at the concentrations indicated. Proliferation was measured by [3H]thymidine incorporation during the last 16∼18 h of a 5-day culture. This is a representative result of three different experiments.
Figure 3
Generation of FluM1 and MART-1-specific CTL using K32/4-1BBL aAPCs. A, CD8 cells from a normal HLA-A2+ donor underwent polyclonal expansion using anti-CD3/4-1BBL resulting in enrichment of FluM1-specific cells to 0.9% by day 10. Cells were then stained with FluM1 tetramers, electronically sorted, collected, and re-expanded using anti-CD3/4-1BBL aAPCs. Ag-specific cell numbers increased from 1.4 × 104 cells on day 10 presort to 2.4 × 105 cells on day 24. The expanded cells demonstrated specificity and functional capacity as evidenced by CD107a expression (bold) and intracellular IFN-γ production (bold) in response to FluM1- but not irrelevant peptide (CAP1)-pulsed T2 targets. Isotype controls are shown in gray. This is representative of more than nine experiments sorted using both CMV- and flu-specific cells. B, Left, Expansion of Mart-1-enriched CTLs using KT32/anti-CD3 aAPCs expressing 4-1BBL, 4-1BBL + CD80, or CD80. Mart-1-specific CD8+ T cell frequency, assessed using tetramer-based flow cytometry, was 10.5% on day 0, and 13.7 and 12.8% on day 9 in the KT32/anti-CD3/4-1BBL and KT32/anti-CD3/4-1BBL/CD80 aAPC-stimulated cultures, respectively. KT32/anti-CD3/CD80 aAPCs did not expand cells after day 4, and no viable CTLs were recovered on day 8, which prevented further phenotype/functional analysis for this condition. Middle, Mart-1 CTL were tested for cytotoxicity using a 4-h chromium release assay on day 9 post-stimulation with either KT32/anti-CD3/4-1BBL (square) or KT32/anti-CD3/4-1BBL/CD80 (diamond) aAPCs. Cr-labeled T2 targets were pulsed with Mart-1 (solid) or control irrelevant peptide (open). There were insufficient expanded CTLs to study cells cocultured with KT32 aCD3/CD80 aAPCs. Right, Mart-1-enriched CTLs were repetitively stimulated (days 0, 9, and 14) using KT32/anti-CD3/IL-15/4-1BBL/CD80 aAPCs for long-term expansion. Mart-1-specific cells ranged from 10 to 20% during culture (data not shown).
Figure 4
Selective expansion of Ag-specific CTL using K32/4-1BBL/HLA-A2-Fc aAPCs. A, CD8 cells were stimulated using K32/4-1BBL/pp65-HLA-A2-Fc, K32/anti-CD28/pp65-HLA-A2-Fc, or K32/pp65-HLA-A2-Fc. On day 22 after three stimulations, the cells were harvested, counted, and analyzed by FACS. K32/4-1BBL/pp65 aAPCs expanded Ag-specific cells but not cells binding an irrelevant tetramer while K32/anti-CD28pp65 and K32/pp65 did not expand Ag-specific cells. B, K32/4-1BBL/pp65, but not K32/anti-CD28/pp65, expanded CTL demonstrate Ag-specific cytotoxicity as assessed by CD107a expression. CD8+ cells stimulated with K32/pp65 did not produce sufficient cell numbers to perform the assay. This is a representative result of three experiments using Ag-specific expansion from different HLA-A2+ donors.
Figure 5
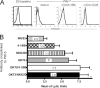
4-1BB signaling augments cytolytic activity of expanded CTL. A, Anti-CD3/4-1BBL aAPC-expanded CD8+ T cells up-regulate NKG2D expression. Purified human CD8 T cells were unstimulated, or stimulated with anti-CD3/4-1BBL or anti-CD3/anti-CD28 aAPC, then assessed for NKG2D expression (bold) on days 0 and 7. Isotype control is shown in gray. This is representative of three experiments from different donors. B, Cytolytic function of human CD8 T cells stimulated with anti-CD3/4-1BBL aAPCs 7 days prior was assayed using 51Cr-labeled P815 target cells cross-linked with the Abs indicated for 4 h. The bar graph is from eight separate experiments with individual donors. Resting CD8 cells and anti-CD3/anti-CD28 aAPC CD8 cells showed no detectable cytolytic activity (data not shown). CD3 and NKG2D signaling alone augment cytolytic function of anti-CD3/4-1BBL aAPC-expanded CD8+ T cells (p < 0.05) whereas cytolytic function after 4-1BB signaling alone was not significantly different than observed with anti-MHC class I. Combining 4-1BB and CD3 signaling or NKG2D and CD3 signaling significantly increases cytolytic activity compared with CD3 signaling alone (**, p = 0.008).
Similar articles
- 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy.
Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, Watts TH. Cannons JL, et al. J Immunol. 2001 Aug 1;167(3):1313-24. doi: 10.4049/jimmunol.167.3.1313. J Immunol. 2001. PMID: 11466348 - A short CD3/CD28 costimulation combined with IL-21 enhance the generation of human memory stem T cells for adoptive immunotherapy.
Alvarez-Fernández C, Escribà-Garcia L, Vidal S, Sierra J, Briones J. Alvarez-Fernández C, et al. J Transl Med. 2016 Jul 19;14(1):214. doi: 10.1186/s12967-016-0973-y. J Transl Med. 2016. PMID: 27435312 Free PMC article. - Transient stimulation expands superior antitumor T cells for adoptive therapy.
Kagoya Y, Nakatsugawa M, Ochi T, Cen Y, Guo T, Anczurowski M, Saso K, Butler MO, Hirano N. Kagoya Y, et al. JCI Insight. 2017 Jan 26;2(2):e89580. doi: 10.1172/jci.insight.89580. JCI Insight. 2017. PMID: 28138559 Free PMC article. - Memory T cells need CD28 costimulation to remember.
Boesteanu AC, Katsikis PD. Boesteanu AC, et al. Semin Immunol. 2009 Apr;21(2):69-77. doi: 10.1016/j.smim.2009.02.005. Epub 2009 Mar 5. Semin Immunol. 2009. PMID: 19268606 Free PMC article. Review. - Costimulation signals for memory CD8+ T cells during viral infections.
Duttagupta PA, Boesteanu AC, Katsikis PD. Duttagupta PA, et al. Crit Rev Immunol. 2009;29(6):469-86. doi: 10.1615/critrevimmunol.v29.i6.20. Crit Rev Immunol. 2009. PMID: 20121696 Free PMC article. Review.
Cited by
- Preclinical Evaluation of Novel Folate Receptor 1-Directed CAR T Cells for Ovarian Cancer.
Daigre J, Martinez-Osuna M, Bethke M, Steiner L, Dittmer V, Krischer K, Bleilevens C, Brauner J, Kopatz J, Grundmann MD, Praveen P, Eckardt D, Bosio A, Herbel C. Daigre J, et al. Cancers (Basel). 2024 Jan 12;16(2):333. doi: 10.3390/cancers16020333. Cancers (Basel). 2024. PMID: 38254822 Free PMC article. - Finding Balance: T cell Regulatory Receptor Expression during Aging.
Cavanagh MM, Qi Q, Weyand CM, Goronzy JJ. Cavanagh MM, et al. Aging Dis. 2011 Oct;2(5):398-413. Epub 2011 Oct 28. Aging Dis. 2011. PMID: 22396890 Free PMC article. - Cancer cell targeting by CAR-T cells: A matter of stemness.
D'Accardo C, Porcelli G, Mangiapane LR, Modica C, Pantina VD, Roozafzay N, Di Franco S, Gaggianesi M, Veschi V, Lo Iacono M, Todaro M, Turdo A, Stassi G. D'Accardo C, et al. Front Mol Med. 2022 Dec 13;2:1055028. doi: 10.3389/fmmed.2022.1055028. eCollection 2022. Front Mol Med. 2022. PMID: 39086964 Free PMC article. Review. - Engineering lymphocyte subsets: tools, trials and tribulations.
June CH, Blazar BR, Riley JL. June CH, et al. Nat Rev Immunol. 2009 Oct;9(10):704-16. doi: 10.1038/nri2635. Nat Rev Immunol. 2009. PMID: 19859065 Free PMC article. Review. - Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma.
Raj D, Yang MH, Rodgers D, Hampton EN, Begum J, Mustafa A, Lorizio D, Garces I, Propper D, Kench JG, Kocher HM, Young TS, Aicher A, Heeschen C. Raj D, et al. Gut. 2019 Jun;68(6):1052-1064. doi: 10.1136/gutjnl-2018-316595. Epub 2018 Aug 18. Gut. 2019. PMID: 30121627 Free PMC article.
References
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. - PubMed
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. - PubMed
- Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. - PubMed
- Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, Sabat R, Babel N, Jones JF, Webster-Cyriaque J, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100:4059–4066. - PubMed
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood. 2005;105:1898–1904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous