Labeling of mesenchymal stem cells by bioconjugated quantum dots - PubMed (original) (raw)
Labeling of mesenchymal stem cells by bioconjugated quantum dots
Bhranti S Shah et al. Nano Lett. 2007 Oct.
Abstract
Long-term labeling of stem cells during self-replication and differentiation benefits investigations of development and tissue regeneration. We report the labeling of human mesenchymal stem cells (hMSCs) with RGD-conjugated quantum dots (QDs) during self-replication, and multilineage differentiations into osteogenic, chondrogenic, and adipogenic cells. QD-labeled hMSCs remained viable as unlabeled hMSCs from the same subpopulation. These findings suggest the use of bioconjugated QDs as an effective probe for long-term labeling of stem cells.
Figures
Figure 1
Human mesenchymal stem cells (hMSCs) labeled with bioconjugated quantum dots (QDs) undergo proliferation up to the tested 22 days. hMSCs after overnight incubation with bioconjugated QDs (30 nM) (a–a2) Following the removal of extracellular QDs, QD-labeled hMSCs and unlabeled hMSCs of the same subpopulation were continuously cultured for 4, 7, and 22 days (b–b2, c–c2, d–d2, respectively). Scale bar: 30 _μ_m. QDs were internalized in the cytoplasm, even after 22 days of culture-expansion (e–e2), clearly observed in fluorescent (e1) and overlay (e2) images, apparently endocytosed as aggregates. Scale bar: 5 _μ_m.
Figure 2
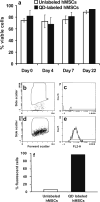
Cell viability and fluorescent cell sorting. Cell viability lacked statistically significant difference between QD-labeled hMSCs and unlabeled hMSCs (a) with or without QD labeling, substantial number of hMSCs remained viable (range: 67% to 93%). Compared to unlabeled hMSCs (6.5%) (b,c), fluorescence-activated sorting shows the yield of QD-labeled hMSCs at 96% (d,e) in the fluorescent range of 605 ± 20 nm with quantitative data shown in (f).
Figure 3
Transwell culture shows a lack of cross-labeling of human mesenchymal stem cells (hMSCs) by bioconjugated quantum dots (QDs). QD-labeled hMSCs were cultured in the insert of a transwell system. The diameter of the insert is 400 nm, much larger than the diameter of QDs in the range of 2–10 nm. Unlabeled hMSCs were cultured underneath in the transwell plate. Whereas QD-labeled hMSCs were observed under fluorescent microscope during the tested 1, 4, and 7 days (a1, b1, and c1), no apparent QD labeling was observed in the unlabeled hMSCs cultured underneath in the same medium (a3, b3, and c3). (a–c) Bright-field images of QD labeled hMSCs; (a2–c2) bright-field image of unlabeled hMSCs. These data suggest that QDs extruded by hMSCs are not taken up by the unlabeled hMSCs up to the tested 7 days of culture. Scale bar: 100 _μ_m.
Figure 4
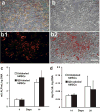
Quantum dot (QD) labeling of human mesenchymal stem cells (hMSCs) during osteogenic differentiation. (a) Expression of alkaline phosphatase (ALP) during osteogenic differentiation of QD-labeled hMSCs. (b–b2) QDs remained in hMSCs during osteogenic differentiation: (b) brightfield image of hMSCs labeled with QDs; (b1) fluorescent image of b1 showing QD labeling; (b3) overlay of (b) and (b1). (c,d) No significant differences in ALP content and calcium production between QD-labeled and unlabeled hMSC-derived osteoblasts, respectively. Scale: 30 _μ_m.
Figure 5
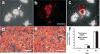
Quantum dot (QD) labeling of human mesenchymal stem cells (hMSCs) during chondrogenic differentiation. (a–c) QD labeling of hMSCs during chondrogenic differentiation in pellet culture ((a) bright-field; (b) fluorescent; (c) overlay). (d,e) Positive alcian blue staining of QD-labeled or unlabeled hMSCs during chondrogenic differentiation. (f) No statistically significant difference in glycosaminoglycan (GAG) content between QD-labeled and unlabeled hMSC-derived chondrocytes. Scale bar: 250 _μ_m.
Figure 6
Quantum dot (QD) labeling of human mesenchymal stem cells (hMSCs) during adipogenic differentiation. (a–c) Formation of intracellular lipid vacuoles in QD-labeled hMSCs during adipogenic differentiation. Arrow points to intracellular lipid vacuole. Scale bar: 50 _μ_m. (d,e) Oil-red O staining showing adipogenesis formation without (d) or with (e) QD labeling. Scale bar: 100 _μ_m. (f) No statistically significant difference in glycerol content between QD-labeled and unlabeled hMSC-derived adipocytes.
Similar articles
- Labeling of mesenchymal stem cells with bioconjugated quantum dots.
Shah BS, Mao JJ. Shah BS, et al. Methods Mol Biol. 2011;680:61-75. doi: 10.1007/978-1-60761-901-7_4. Methods Mol Biol. 2011. PMID: 21153373 - Labeling and imaging of human mesenchymal stem cells with quantum dot bioconjugates during proliferation and osteogenic differentiation in long term.
Shah B, Clark P, Stroscio M, Mao J. Shah B, et al. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:1470-3. doi: 10.1109/IEMBS.2006.260082. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17946892 - Multifunctional Quantum Dot Nanoparticles for Effective Differentiation and Long-Term Tracking of Human Mesenchymal Stem Cells In Vitro and In Vivo.
Li J, Lee WY, Wu T, Xu J, Zhang K, Li G, Xia J, Bian L. Li J, et al. Adv Healthc Mater. 2016 May;5(9):1049-57. doi: 10.1002/adhm.201500879. Epub 2016 Feb 25. Adv Healthc Mater. 2016. PMID: 26919348 - In vivo molecular and cellular imaging with quantum dots.
Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. Gao X, et al. Curr Opin Biotechnol. 2005 Feb;16(1):63-72. doi: 10.1016/j.copbio.2004.11.003. Curr Opin Biotechnol. 2005. PMID: 15722017 Review. - Probing dynamic fluorescence properties of single and clustered quantum dots toward quantitative biomedical imaging of cells.
Kang HG, Tokumasu F, Clarke M, Zhou Z, Tang J, Nguyen T, Hwang J. Kang HG, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 Jan-Feb;2(1):48-58. doi: 10.1002/wnan.62. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010. PMID: 20049830 Review.
Cited by
- Nanoparticles and their potential for application in bone.
Tautzenberger A, Kovtun A, Ignatius A. Tautzenberger A, et al. Int J Nanomedicine. 2012;7:4545-57. doi: 10.2147/IJN.S34127. Epub 2012 Aug 17. Int J Nanomedicine. 2012. PMID: 22923992 Free PMC article. Review. - Stem cell tracking by nanotechnologies.
Villa C, Erratico S, Razini P, Fiori F, Rustichelli F, Torrente Y, Belicchi M. Villa C, et al. Int J Mol Sci. 2010 Mar 12;11(3):1070-81. doi: 10.3390/ijms11031070. Int J Mol Sci. 2010. PMID: 20480000 Free PMC article. Review. - A brief review of cytotoxicity of nanoparticles on mesenchymal stem cells in regenerative medicine.
Liu X, Yang Z, Sun J, Ma T, Hua F, Shen Z. Liu X, et al. Int J Nanomedicine. 2019 May 24;14:3875-3892. doi: 10.2147/IJN.S205574. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31213807 Free PMC article. Review. - Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation.
Walker PA, Shah SK, Harting MT, Cox CS Jr. Walker PA, et al. Dis Model Mech. 2009 Jan-Feb;2(1-2):23-38. doi: 10.1242/dmm.001198. Dis Model Mech. 2009. PMID: 19132123 Free PMC article. Review. - The influence of Aloe vera with mesenchymal stem cells from dental pulp on bone regeneration: characterization and treatment of non-critical defects of the tibia in rats.
Soares IMV, Fernandes GVO, Larissa Cordeiro C, Leite YKPC, Bezerra DO, Carvalho MAM, Carvalho CMRS. Soares IMV, et al. J Appl Oral Sci. 2019;27:e20180103. doi: 10.1590/1678-7757-2018-0103. Epub 2019 Apr 11. J Appl Oral Sci. 2019. PMID: 30994771 Free PMC article.
References
- Fairchild PJ, Nolan KF, Cartland S, Waldmann H. Int Immunopharmacol. 2005;5:13–21. - PubMed
- Prockop D. J Science. 2001;293:211–212. - PubMed
- Parker GC, Nastassova-Kristeva M, Eisenberg LM, Rao MS, Williams MA, Sanberg PR, English D. Stem Cells Dev. 2005;14:463–469. - PubMed
- Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Cytotherapy. 2006;8:315–317. - PubMed
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. Cell Tissue Kinet. 1970;3:393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB009663/EB/NIBIB NIH HHS/United States
- RC2 DE020767-02/DE/NIDCR NIH HHS/United States
- DE15391/DE/NIDCR NIH HHS/United States
- RC2 DE020767/DE/NIDCR NIH HHS/United States
- R01 EB002332/EB/NIBIB NIH HHS/United States
- R01 EB006261/EB/NIBIB NIH HHS/United States
- R01 DE015391/DE/NIDCR NIH HHS/United States
- EB02332/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources