Increased phosphorylation of Akt in triple-negative breast cancers - PubMed (original) (raw)
Increased phosphorylation of Akt in triple-negative breast cancers
Shinobu Umemura et al. Cancer Sci. 2007 Dec.
Abstract
Cells from breast cancers lacking hormone receptors (estrogen receptor [ER], progesterone receptor [PgR]) and human epidermal growth factor receptor (HER) 2 strongly express the cell proliferation marker Ki-67. However, the mechanisms of and stimulus signals involved in cell proliferation of this type of breast cancer are not well understood. The aim of the present study was to examine the characteristics of signal transduction in triple-negative (ER-, PgR-, and HER2-negative) breast cancers. For 44 tumor samples, western blotting analysis was conducted to examine the phosphorylation of HER2, external signal-regulated kinase (ERK)1 and -2 and Akt, and the immunohistochemical phenotypes of the samples with respect to ER and HER2 were also assessed. Phosphorylation of HER2 was detected in 4 of 15 immunohistochemically HER2-positive tumor samples (26.7%). ERK1/2 was more highly phosphorylated in triple-negative breast cancers. Phosphorylation of Akt kinase was significantly higher in triple-negative breast cancers. Triple-negative breast cancers are characterized by increased phosphorylation of Akt kinase. In the present study, we found for the first time that there is a population with a significantly activated Akt pathway in this type of breast cancer.
Figures
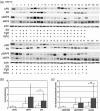
Figure 1
Immunoblotting analysis for human epidermal growth factor receptor (HER) 2, external signal‐regulated kinase (ERK)1/2, and Akt kinases. (a) Protein extracts from 44 tumor samples were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunoblotted using antibodies for phosphorylated and total HER2, ERK1/2, and Akt. M1, M2, and M3 are markers for protein extracts in graded concentrations. (b) Phosphorylated HER2, ERK1/2, and Akt were compared between ER‐, PgR‐, and HER2‐negative cancers (▪), and other types of breast cancers, including ER+ and HER2−, ER+ and HER2+, and ER− and HER2+ cancers (□). Asterisks indicate _P_‐value (0.0023 for the _t_‐test and 0.06 for the Mann–Whitney test). Error bars represent SD. (c) The ratios of phosphorylated and total HER2, ERK1/2, and Akt were also compared between triple‐negative breast cancers (▪) and others (□). Asterisks indicate _P_‐value (0.006 for the _t_‐test).
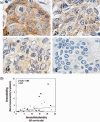
Figure 2
Immunohistochemical analysis for phosphorylated Akt. Immunohistochemical results were evaluated by a combination of immunohistochemical intensity and population. The population of carcinoma‐positive cells with strong (3+), medium (2+), or weak (1+) intensity, or negative cells, were estimated. (b) The results of immunoblotting and immunohistochemical analysis were in good correlation. Solid circles indicate ER−, and PgR− and human epidermal growth factor receptor (HER)2−, and open circles indicate other types of breast cancers, including ER+ and HER2−, ER+ and HER2+, and ER and HER2+.
Similar articles
- Modulating therapeutic effects of the c-Src inhibitor via oestrogen receptor and human epidermal growth factor receptor 2 in breast cancer cell lines.
Fan P, McDaniel RE, Kim HR, Clagett D, Haddad B, Jordan VC. Fan P, et al. Eur J Cancer. 2012 Dec;48(18):3488-98. doi: 10.1016/j.ejca.2012.04.020. Epub 2012 Jun 2. Eur J Cancer. 2012. PMID: 22658320 Free PMC article. - Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1.
Ueda S, Tsuda H, Sato K, Takeuchi H, Shigekawa T, Matsubara O, Hiraide H, Mochizuki H. Ueda S, et al. Cancer Sci. 2006 Jul;97(7):597-604. doi: 10.1111/j.1349-7006.2006.00228.x. Cancer Sci. 2006. PMID: 16827799 Free PMC article. - HER2 as a prognostic factor in breast cancer.
Ménard S, Fortis S, Castiglioni F, Agresti R, Balsari A. Ménard S, et al. Oncology. 2001;61 Suppl 2:67-72. doi: 10.1159/000055404. Oncology. 2001. PMID: 11694790 Review. - In search of triple-negative DCIS: tumor-type dependent model of breast cancer progression from DCIS to the invasive cancer.
Kurbel S. Kurbel S. Tumour Biol. 2013 Feb;34(1):1-7. doi: 10.1007/s13277-012-0602-1. Epub 2012 Dec 4. Tumour Biol. 2013. PMID: 23208673 Review.
Cited by
- The Multiple Functions of HB-EGF in Female Reproduction and Related Cancer: Molecular Mechanisms and Targeting Strategies.
Zhang Y, Tang L, Liu H, Cheng Y. Zhang Y, et al. Reprod Sci. 2024 Sep;31(9):2588-2603. doi: 10.1007/s43032-024-01454-6. Epub 2024 Feb 29. Reprod Sci. 2024. PMID: 38424408 Review. - NO to breast: when, why and why not?
Pervin S, Chaudhuri G, Singh R. Pervin S, et al. Curr Pharm Des. 2010;16(4):451-62. doi: 10.2174/138161210790232130. Curr Pharm Des. 2010. PMID: 20236074 Free PMC article. Review. - PKIB expression strongly correlated with phosphorylated Akt expression in breast cancers and also with triple-negative breast cancer subtype.
Dabanaka K, Chung S, Nakagawa H, Nakamura Y, Okabayashi T, Sugimoto T, Hanazaki K, Furihata M. Dabanaka K, et al. Med Mol Morphol. 2012 Dec;45(4):229-33. doi: 10.1007/s00795-011-0565-0. Epub 2012 Dec 7. Med Mol Morphol. 2012. PMID: 23224602 - Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells.
Marty B, Maire V, Gravier E, Rigaill G, Vincent-Salomon A, Kappler M, Lebigot I, Djelti F, Tourdès A, Gestraud P, Hupé P, Barillot E, Cruzalegui F, Tucker GC, Stern MH, Thiery JP, Hickman JA, Dubois T. Marty B, et al. Breast Cancer Res. 2008;10(6):R101. doi: 10.1186/bcr2204. Epub 2008 Dec 3. Breast Cancer Res. 2008. PMID: 19055754 Free PMC article. - Rosemary Extract Inhibits Proliferation, Survival, Akt, and mTOR Signaling in Triple-Negative Breast Cancer Cells.
Jaglanian A, Tsiani E. Jaglanian A, et al. Int J Mol Sci. 2020 Jan 27;21(3):810. doi: 10.3390/ijms21030810. Int J Mol Sci. 2020. PMID: 32012648 Free PMC article.
References
- Gaben AM, Saucier C, Bedin M et al . Mitogenic activity of estrogens in human breast cancer cells does not rely on direct induction of mitogen‐activated protein kinase/extracellularly regulated kinase or phosphatidylinositol 3‐kinase. Mol Endocrinol 2004; 18: 2700–13. - PubMed
- Dubik D, Shiu RP. Mechanism of estrogen activation of c‐myc oncogene expression. Oncogene 1992; 7: 1587–94. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous