Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection - PubMed (original) (raw)
Review
Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection
Susan M Kaech et al. Immunity. 2007 Sep.
Abstract
Heterogeneity is a hallmark of the adaptive immune system. This is most evident in the enormous diversity of B and T cell antigen receptors. There is also heterogeneity within antiviral T cell populations, and subsets of effector and memory T cells now permeate our thinking about specialization of T cell responses to pathogens. It has been less clear, however, how heterogeneity in developing virus-specific effector and memory T cells is related to cell-fate decisions in the immune response, such as the generation long-lived memory T cells. Here we discuss recent findings that might help redefine how heterogeneity in antiviral T cell populations gives rise to T cell subsets with short- and long-lived cell fates.
Figures
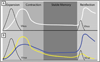
Figure 1. A Homogenous and Heterogeneous View on Antiviral Memory T Cell Development
(A) T cells clonally expand and homogeneously differentiate into effector T cells, of which most die, but some persist to become long-lived memory T cells. Upon reinfection, memory T cells re-expand and control infection faster than 1° infection. (B) Two populations of effector T cells form with different memory T cell potential: short-lived effector T cells (SLECs; yellow line) that do not gain memory T cell potential, and memory precursor effector cells (MPECs; blue line) that do. Upon reinfection, it is primarily the descendents of MPECs that participate in secondary responses because of their enhanced proliferative capacity.
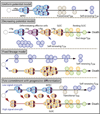
Figure 2. Models of Effector and Memory Cell-Fate Decisions during Acute Viral Infection
Model 1—uniform potential. All activated effector cells develop equal potential to become MPECs (aqua cells), but extrinsic factors limit the number of memory T cells generated. MPECs give rise to transitional TEM cells (gray) that convert into self-renewing TCM cells (dark blue). Model 2—decreasing potential. Shorter durations of antigenic stimulation favor MPECs that give rise to TCM cells. Longer stimulation promotes terminal differentiation of SLECs (yellow) and end-stage TEM cells (green) that decline over time. Model 3—fixed lineage. Upon activation, naive T cells develop into either SLECs or fully mature memory T cells. In this model, cells might bypass an effector stage and develop directly into self-renewing TCM. Model 4—fate commitment with progressive differentiation. According to the strength of signal, either an MPEC or SLEC fate will be adopted early after activation. The MPECs acquire effector functions, but remain multipotent (e.g., can still become memory T cells or SLECs). Most SLECs die, but some persist with a limited lifespan as an end-stage TEM cell. MPECs survive, and give rise to transitional TEM cells that progressively mature into long-lived TCM cells.
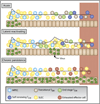
Figure 3. Differential Effects of Acute, Latent or Chronic Viral Infections on Memory T Cell Differentiation
Viral infections fall into three categories based on the duration and pattern of viral infection—(1) acute, (2) latent with reactivation, and (3) chronic or persistent. Primary acute infection generates SLECs (yellow) and MPECs (aqua). For a description of the cell types, see Figure 2. The majority of SLECs die, but some persist for finite intervals (TEM cells; green). In contrast, MPECs survive and progressively mature from transitional TEM cells (light blue) into protective TCM cells (dark blue). During latent or reactivating viral infections, a mixed population with a variety of effector and memory differentiate states is formed. Typically, there will be more SLECs and TEM cells than after acute infection. The abundance of secondary MPECs, resting TCM cells, or transitional TEM cells will likely depend on the frequency and degree of viral reactivation. During persistent or chronic viral infections, with high viremia, T cells can undergo altered differentiation and become exhausted (red). According to the type of infection, the snap-shot of effector and memory T cells present (outlined in red box) is likely to be different. See Table 1 for detailed descriptions of common attributes.
Similar articles
- Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections.
Philip M, Schietinger A. Philip M, et al. Curr Opin Immunol. 2019 Jun;58:98-103. doi: 10.1016/j.coi.2019.04.014. Epub 2019 Jun 8. Curr Opin Immunol. 2019. PMID: 31181510 Free PMC article. Review. - CD8 T-cell memory differentiation during acute and chronic viral infections.
Kalia V, Sarkar S, Ahmed R. Kalia V, et al. Adv Exp Med Biol. 2010;684:79-95. doi: 10.1007/978-1-4419-6451-9_7. Adv Exp Med Biol. 2010. PMID: 20795542 - One naive T cell, multiple fates in CD8+ T cell differentiation.
Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, Zehn D, Bevan MJ, Schepers K, Schumacher TN. Gerlach C, et al. J Exp Med. 2010 Jun 7;207(6):1235-46. doi: 10.1084/jem.20091175. Epub 2010 May 17. J Exp Med. 2010. PMID: 20479114 Free PMC article. - Developmental Origin Governs CD8+ T Cell Fate Decisions during Infection.
Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, Watson NB, Nzingha K, Yee Mon KJ, Peng SA, Grimson A, Davenport MP, Rudd BD. Smith NL, et al. Cell. 2018 Jun 28;174(1):117-130.e14. doi: 10.1016/j.cell.2018.05.029. Epub 2018 Jun 14. Cell. 2018. PMID: 29909981 - Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells.
Enamorado M, Khouili SC, Iborra S, Sancho D. Enamorado M, et al. Front Immunol. 2018 Jul 31;9:1751. doi: 10.3389/fimmu.2018.01751. eCollection 2018. Front Immunol. 2018. PMID: 30108585 Free PMC article. Review.
Cited by
- CD4(+) T-cell dependence of primary CD8(+) T-cell response against vaccinia virus depends upon route of infection and viral dose.
Hu Z, Molloy MJ, Usherwood EJ. Hu Z, et al. Cell Mol Immunol. 2016 Jan;13(1):82-93. doi: 10.1038/cmi.2014.128. Epub 2014 Dec 29. Cell Mol Immunol. 2016. PMID: 25544501 Free PMC article. - Influenza and Memory T Cells: How to Awake the Force.
Spitaels J, Roose K, Saelens X. Spitaels J, et al. Vaccines (Basel). 2016 Oct 13;4(4):33. doi: 10.3390/vaccines4040033. Vaccines (Basel). 2016. PMID: 27754364 Free PMC article. Review. - Temporal expression of microRNA cluster miR-17-92 regulates effector and memory CD8+ T-cell differentiation.
Wu T, Wieland A, Araki K, Davis CW, Ye L, Hale JS, Ahmed R. Wu T, et al. Proc Natl Acad Sci U S A. 2012 Jun 19;109(25):9965-70. doi: 10.1073/pnas.1207327109. Epub 2012 Jun 4. Proc Natl Acad Sci U S A. 2012. PMID: 22665768 Free PMC article. - Understanding Subset Diversity in T Cell Memory.
Jameson SC, Masopust D. Jameson SC, et al. Immunity. 2018 Feb 20;48(2):214-226. doi: 10.1016/j.immuni.2018.02.010. Immunity. 2018. PMID: 29466754 Free PMC article. Review. - Genomic profiling of intestinal T-cell receptor repertoires in inflammatory bowel disease.
Saravanarajan K, Douglas AR, Ismail MS, Omorogbe J, Semenov S, Muphy G, O'Riordan F, McNamara D, Nakagome S. Saravanarajan K, et al. Genes Immun. 2020 Feb;21(2):109-118. doi: 10.1038/s41435-020-0092-x. Epub 2020 Feb 7. Genes Immun. 2020. PMID: 32029881
References
- Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272:54–60. - PubMed
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. - PubMed
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. - PubMed
- Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40–CD40 ligand: Antibodies provide the key. Eur. J. Immunol. 2004;34:317–326. - PubMed
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 2005a;175:4686–4696. - PubMed
Publication types
MeSH terms
Grants and funding
- 1004313/WT_/Wellcome Trust/United Kingdom
- AI066232/AI/NIAID NIH HHS/United States
- AI077075/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R21 AI077075/AI/NIAID NIH HHS/United States
- R01 AI066232/AI/NIAID NIH HHS/United States
- AI071309/AI/NIAID NIH HHS/United States
- R37 AI066232/AI/NIAID NIH HHS/United States
- R01 AI071309/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials