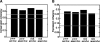Systematic condition-dependent annotation of metabolic genes - PubMed (original) (raw)
Systematic condition-dependent annotation of metabolic genes
Tomer Shlomi et al. Genome Res. 2007 Nov.
Abstract
The task of deriving a functional annotation for genes is complex as their involvement in various processes depends on multiple factors such as environmental conditions and genetic backup mechanisms. This study employs a large-scale model of the metabolism of Saccharomyces cerevisiae to investigate the function of yeast genes and derive a condition-dependent annotation (CDA) for their involvement in major metabolic processes under various genetic and environmental conditions. The resulting CDA is validated on a large scale and is shown to be superior to the corresponding Gene Ontology (GO) annotation, by showing that genes annotated with the same CDA term tend to be more coherently conserved in evolution and display greater expression coherency than those annotated with the same GO term. The CDA gives rise to new kinds of functional condition-dependent metabolic pathways, some of which are described and further examined via substrate auxotrophy measurements of knocked-out strains. The CDA presented is likely to serve as a new reference source for metabolic gene annotation.
Figures
Figure 1.
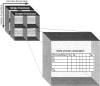
Condition-dependent annotation (CDA). A schematic representation of the CDA as a set of associations between genes and processes under various conditions. The basic element is a table specifying an association between genes and processes. This condition-dependent association table is the content of each entry of the CDA. These entries are spanned in turn by two dimensions representing growth media and the availability of oxygen (but additional dimensions can be added in principle in accordance with the data available). We consider two annotation systems, for single- and for double-knockouts simulations.
Figure 2.
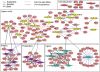
Novel CDA predictions of known GO annotations. Nodes represent GO terms (top curve) and CDA terms (bottom curve). An edge between GO term x and CDA term y represents a set of novel CDA associations of genes annotated in GO as involved in term x, and annotated with term y in CDA. The width of the edge represents the set size. Blue and red edges represent annotations obtained with single and double knockouts, respectively.
Figure 3.
A network view of CDA in single knockout, poor media conditions, in aerobic and anaerobic conditions. Genes are marked with red circular nodes, and process terms with colored diamond nodes. Edges connecting between genes and processes denote annotation associations. Dotted lines represent annotations that are also present in GO. Wide edges represent an essential contribution of a gene to a process. Blue, red, and black edges represent contribution under aerobic, anaerobic, and both conditions, respectively. Note that several CDA annotations (e.g., in the pyrimidine biosynthesis cluster) have corresponding GO annotations that are highly nonspecific (Methods) and are hence considered here as novel.
Figure 4.
The conservation (A) and expression (B) coherency of the CDA under different conditions. The black and gray lines represent the coherency score obtained for a random annotation and for GO, respectively.
Figure 5.
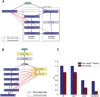
Functional pathways of alanine and proline biosynthesis as reflected by CDA. (A) A functional pathway of alanine biosynthesis under aerobic and anaerobic poor media. Rectangular nodes represent metabolic reactions, specifying names of the coding genes and names of the reactions. The circular node represents the metabolite alanine. Blue edges represent physical interactions between enzymes, in the form of a metabolite that is the product of one enzyme and the substrate of the other. Red edges represent genetic interactions between genes that are specific to alanine biosynthesis. (B) A functional pathway of proline biosynthesis in aerobic minimal media condition. (C) Proline auxotrophy experiment for the CAR2 and PRO2 single deletion, and CAR2/PRO2 double deletion strains. The experiments show a significant drop in maximum optical density (OD) for the CAR2/PRO2 double mutant strain when proline is removed from the growth medium. The results show the existence of a synthetic sick interaction between CAR2 and PRO2 in minimal media that lacks proline.
Similar articles
- AVID: an integrative framework for discovering functional relationships among proteins.
Jiang T, Keating AE. Jiang T, et al. BMC Bioinformatics. 2005 Jun 1;6:136. doi: 10.1186/1471-2105-6-136. BMC Bioinformatics. 2005. PMID: 15929793 Free PMC article. - Combining multisource information through functional-annotation-based weighting: gene function prediction in yeast.
Ray SS, Bandyopadhyay S, Pal SK. Ray SS, et al. IEEE Trans Biomed Eng. 2009 Feb;56(2):229-36. doi: 10.1109/TBME.2008.2005955. Epub 2008 Sep 30. IEEE Trans Biomed Eng. 2009. PMID: 19272921 - Systematic identification and correction of annotation errors in the genetic interaction map of Saccharomyces cerevisiae.
Atias N, Kupiec M, Sharan R. Atias N, et al. Nucleic Acids Res. 2016 Mar 18;44(5):e50. doi: 10.1093/nar/gkv1284. Epub 2015 Nov 23. Nucleic Acids Res. 2016. PMID: 26602688 Free PMC article. - Gene Ontology annotation status of the fission yeast genome: preliminary coverage approaches 100%.
Aslett M, Wood V. Aslett M, et al. Yeast. 2006 Oct 15;23(13):913-9. doi: 10.1002/yea.1420. Yeast. 2006. PMID: 17072883 Review. - Contributions of Saccharomyces cerevisiae to understanding mammalian gene function and therapy.
Zhang N, Bilsland E. Zhang N, et al. Methods Mol Biol. 2011;759:501-23. doi: 10.1007/978-1-61779-173-4_28. Methods Mol Biol. 2011. PMID: 21863505 Review.
Cited by
- The limitations of phenotype prediction in metabolism.
Yubero P, Lavin AA, Poyatos JF. Yubero P, et al. PLoS Comput Biol. 2023 Nov 10;19(11):e1011631. doi: 10.1371/journal.pcbi.1011631. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37948461 Free PMC article. - The microRNA Cargo of Human Vaginal Extracellular Vesicles Differentiates Parasitic and Pathobiont Infections from Colonization by Homeostatic Bacteria.
Cezar-de-Mello PFT, Ryan S, Fichorova RN. Cezar-de-Mello PFT, et al. Microorganisms. 2023 Feb 21;11(3):551. doi: 10.3390/microorganisms11030551. Microorganisms. 2023. PMID: 36985125 Free PMC article. - Exploring gene knockout strategies to identify potential drug targets using genome-scale metabolic models.
Paul A, Anand R, Karmakar SP, Rawat S, Bairagi N, Chatterjee S. Paul A, et al. Sci Rep. 2021 Jan 8;11(1):213. doi: 10.1038/s41598-020-80561-1. Sci Rep. 2021. PMID: 33420254 Free PMC article. - Genetic buffering and potentiation in metabolism.
Poyatos JF. Poyatos JF. PLoS Comput Biol. 2020 Sep 14;16(9):e1008185. doi: 10.1371/journal.pcbi.1008185. eCollection 2020 Sep. PLoS Comput Biol. 2020. PMID: 32925942 Free PMC article. - Alleles of a gene differ in pleiotropy, often mediated through currency metabolite production, in E. coli and yeast metabolic simulations.
Alzoubi D, Desouki AA, Lercher MJ. Alzoubi D, et al. Sci Rep. 2018 Nov 22;8(1):17252. doi: 10.1038/s41598-018-35092-1. Sci Rep. 2018. PMID: 30467356 Free PMC article.
References
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Dolinski K., Dwight S.S., Eppig J.T., Dwight S.S., Eppig J.T., Eppig J.T., et al. Gene Ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. - PMC - PubMed
- Ball C.A., Awad I.A., Demeter J., Gollub J., Hebert J.M., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Awad I.A., Demeter J., Gollub J., Hebert J.M., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Demeter J., Gollub J., Hebert J.M., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Gollub J., Hebert J.M., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Hebert J.M., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Hernandez-Boussard T., Jin H., Matese J.C., Nitzberg M., Wymore F., Jin H., Matese J.C., Nitzberg M., Wymore F., Matese J.C., Nitzberg M., Wymore F., Nitzberg M., Wymore F., Wymore F., et al. The Stanford Microarray Database accommodates additional microarray platforms and data formats. Nucleic Acids Res. 2005;33:D580–D582. doi: 10.1093/nar/gki006. - DOI - PMC - PubMed
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C., Denouel A., Lacroute F., Cullin C., Lacroute F., Cullin C., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. - DOI - PMC - PubMed
- Brown J.A., Sherlock G., Myers C.L., Burrows N.M., Deng C., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., Sherlock G., Myers C.L., Burrows N.M., Deng C., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., Myers C.L., Burrows N.M., Deng C., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., Burrows N.M., Deng C., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., Deng C., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., Wu H.I., McCann K.E., Troyanskaya O.G., Brown J.M., McCann K.E., Troyanskaya O.G., Brown J.M., Troyanskaya O.G., Brown J.M., Brown J.M. Global analysis of gene function in yeast by quantitative phenotypic profiling. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100043. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases