The cyclic AMP cascade is altered in the fragile X nervous system - PubMed (original) (raw)
The cyclic AMP cascade is altered in the fragile X nervous system
Daniel J Kelley et al. PLoS One. 2007.
Abstract
Fragile X syndrome (FX), the most common heritable cause of mental retardation and autism, is a developmental disorder characterized by physical, cognitive, and behavioral deficits. FX results from a trinucleotide expansion mutation in the fmr1 gene that reduces levels of fragile X mental retardation protein (FMRP). Although research efforts have focused on FMRP's impact on mGluR signaling, how the loss of FMRP leads to the individual symptoms of FX is not known. Previous studies on human FX blood cells revealed alterations in the cyclic adenosine 3', 5'-monophosphate (cAMP) cascade. We tested the hypothesis that cAMP signaling is altered in the FX nervous system using three different model systems. Induced levels of cAMP in platelets and in brains of fmr1 knockout mice are substantially reduced. Cyclic AMP induction is also significantly reduced in human FX neural cells. Furthermore, cAMP production is decreased in the heads of FX Drosophila and this defect can be rescued by reintroduction of the dfmr gene. Our results indicate that a robust defect in cAMP production in FX is conserved across species and suggest that cAMP metabolism may serve as a useful biomarker in the human disease population. Reduced cAMP induction has implications for the underlying causes of FX and autism spectrum disorders. Pharmacological agents known to modulate the cAMP cascade may be therapeutic in FX patients and can be tested in these models, thus supplementing current efforts centered on mGluR signaling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
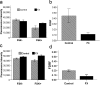
Figure 1. cAMP production is decreased in FX mice.
Platelets (Control n = 3, FX n = 5) and cortical membranes (n = 3 for each group) from wildtype and fmr1 knockout mice were stimulated with forskolin and levels of cAMP were measured (each n averaged in triplicate). Raw fluorescence (RF) is an inverse index of cAMP levels. The change in cAMP production was assessed using the fractional decrease in RF (FDRF) = (No Forskolin−Forskolin)/(No Forskolin). (a) Mouse platelet RF is comparable in the absence of forskolin [t(6) = 0.99; p = 0.36] but significantly different between groups (GroupxStimulus:[F(1,12) = 7.56;p = 0.018]) in the presence of forskolin [t(6) = −2.62; p = 0.040]. (b) Mouse platelet FDRF shows reduced cAMP induction in FX platelets [t(6) = 3.46; p = 0.014]. (c) Mouse cortex RF is comparable between groups in the absence [t(4) = 1.22;p = 0.29] or presence [t(4) = −1.92;p = 0.13] of forskolin. (d) Mouse cortex FDRF shows reduced FX cAMP induction [t(4) = 3.01; p = 0.04]. Error bars are SEM.
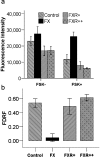
Figure 2. cAMP production is decreased in FX fly.
Head membranes from wildtype, FX, FXR+, FXR++ flies were stimulated with forskolin and levels of cAMP were measured. Raw fluorescence (RF) is an inverse index of cAMP levels. The change in cAMP production was assessed using the fractional decrease in RF (FDRF) = (No Forskolin−Forskolin)/(No Forskolin). (a) RF-based cAMP measures in the fly head (Control n = 10, FX n = 3, FXR+ n = 6; FXR++ n = 6 sets of 20 heads) are comparable between groups in the absence of forskolin [F(3,21) = 1.96; p = 0.15], but not its presence [F(3,21) = 6.23;p = 0.003], since RF intensity in FX flies is larger than the others [t(21) = −4.10;p = 0.000]. (b) Fly head FDRF shows reduced FX cAMP induction relative to control membranes [FDRF:t(21) = 3.85;p = 0.001] and to the FX flies rescued with a single or multiple transgenes [FXR+FDRF:t(21) = 3.23;p = 0.004; FXR++FDRF:t(21) = 4.13;p = 0.000]. Error bars are SEM.
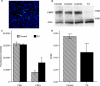
Figure 3. cAMP production is decreased in human FX neural cells.
Unaffected and FX human neural cells were stimulated with forskolin and levels of cAMP were measured. Raw fluorescence (RF) is an inverse index of cAMP levels. The change in cAMP production was assessed using the fractional decrease in RF (FDRF) = (No Forskolin−Forskolin)/(No Forskolin). (a) Neurons (MAP2, green) are present among cells (Hoechst stain, nuclei in blue) in 9 week FX cultures. (b) Extracts of human neural cells were immunoblotted with antibodies to FMRP and actin. FX cells (FX) have reduced levels of FMRP compared to 3 controls (control 1, 2, 3). Similar levels of actin are detected in each sample. (c) Human neural cell RF (2 Controls, each with n = 3; FX, n = 3) is comparable between groups in forskolin's absence [t(6) = 0.82; p = 0.44] but FX cAMP production is lower than controls in the presence of forskolin [RF: t(6) = −3.40; p = 0.015]. (d) Human neural cell FDRF was reduced in FX [t(6) = 3.43; p = 0.01]. Error bars are SEM for FX and pooled SEM for controls. (Pooled SEM)2 = SEM1 2+SEM2 2−2*SEM1*SEM2.
Similar articles
- Prefrontal Cortex Dysfunction in Fragile X Mice Depends on the Continued Absence of Fragile X Mental Retardation Protein in the Adult Brain.
Siegel JJ, Chitwood RA, Ding JM, Payne C, Taylor W, Gray R, Zemelman BV, Johnston D. Siegel JJ, et al. J Neurosci. 2017 Aug 2;37(31):7305-7317. doi: 10.1523/JNEUROSCI.0571-17.2017. Epub 2017 Jun 26. J Neurosci. 2017. PMID: 28652410 Free PMC article. - The cyclic AMP phenotype of fragile X and autism.
Kelley DJ, Bhattacharyya A, Lahvis GP, Yin JC, Malter J, Davidson RJ. Kelley DJ, et al. Neurosci Biobehav Rev. 2008 Oct;32(8):1533-43. doi: 10.1016/j.neubiorev.2008.06.005. Epub 2008 Jun 17. Neurosci Biobehav Rev. 2008. PMID: 18601949 Free PMC article. Review. - Synaptic vesicle dynamic changes in a model of fragile X.
Broek JAC, Lin Z, de Gruiter HM, van 't Spijker H, Haasdijk ED, Cox D, Ozcan S, van Cappellen GWA, Houtsmuller AB, Willemsen R, de Zeeuw CI, Bahn S. Broek JAC, et al. Mol Autism. 2016 Mar 1;7:17. doi: 10.1186/s13229-016-0080-1. eCollection 2016. Mol Autism. 2016. PMID: 26933487 Free PMC article. - Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures.
Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, Cheng X, Warren ST, Klyachko VA. Myrick LK, et al. Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):949-56. doi: 10.1073/pnas.1423094112. Epub 2015 Jan 5. Proc Natl Acad Sci U S A. 2015. PMID: 25561520 Free PMC article. - The fragile X syndrome: exploring its molecular basis and seeking a treatment.
Bardoni B, Davidovic L, Bensaid M, Khandjian EW. Bardoni B, et al. Expert Rev Mol Med. 2006 Apr 21;8(8):1-16. doi: 10.1017/S1462399406010751. Expert Rev Mol Med. 2006. PMID: 16626504 Review.
Cited by
- Cognitive Enhancement in Infants Associated with Increased Maternal Fruit Intake During Pregnancy: Results from a Birth Cohort Study with Validation in an Animal Model.
Bolduc FV, Lau A, Rosenfelt CS, Langer S, Wang N, Smithson L, Lefebvre D, Alexander RT, Dickson CT, Li L, Becker AB, Subbarao P, Turvey SE, Pei J, Sears MR, Mandhane PJ; CHILD Study Investigators. Bolduc FV, et al. EBioMedicine. 2016 Jun;8:331-340. doi: 10.1016/j.ebiom.2016.04.025. Epub 2016 Apr 22. EBioMedicine. 2016. PMID: 27428442 Free PMC article. - Temporally and Spatially Localized PKA Activity within Learning and Memory Circuitry Regulated by Network Feedback.
Sears JC, Broadie K. Sears JC, et al. eNeuro. 2022 Apr 1;9(2):ENEURO.0450-21.2022. doi: 10.1523/ENEURO.0450-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35301221 Free PMC article. - GABAergic circuit dysfunction in the Drosophila Fragile X syndrome model.
Gatto CL, Pereira D, Broadie K. Gatto CL, et al. Neurobiol Dis. 2014 May;65:142-59. doi: 10.1016/j.nbd.2014.01.008. Epub 2014 Jan 12. Neurobiol Dis. 2014. PMID: 24423648 Free PMC article. - Targeted Treatments for Fragile X Syndrome.
Johnson D, Clark C, Hagerman R. Johnson D, et al. Adv Neurobiol. 2023;30:225-253. doi: 10.1007/978-3-031-21054-9_10. Adv Neurobiol. 2023. PMID: 36928853 - Activation of Serotonin 5-HT7 Receptors Modulates Hippocampal Synaptic Plasticity by Stimulation of Adenylate Cyclases and Rescues Learning and Behavior in a Mouse Model of Fragile X Syndrome.
Costa L, Sardone LM, Bonaccorso CM, D'Antoni S, Spatuzza M, Gulisano W, Tropea MR, Puzzo D, Leopoldo M, Lacivita E, Catania MV, Ciranna L. Costa L, et al. Front Mol Neurosci. 2018 Oct 2;11:353. doi: 10.3389/fnmol.2018.00353. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30333723 Free PMC article.
References
- Hagerman RJ. The Physical and Behavioral Phenotype. In: Hagerman PJ, editor. Fragile X Syndrome: Diagnosis, Treatment, and Research. The Johns Hopkins University Press; 2002. pp. 3–87.
- Kremer EJ, Pritchard M, Lynch M, Yu S, Holman K, et al. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252:1711–1714. - PubMed
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. - PubMed
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. - PubMed
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- R01 MH067774/MH/NIMH NIH HHS/United States
- T32 GM008692/GM/NIGMS NIH HHS/United States
- P30 HD03352/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases