A visual pathway links brain structures active during magnetic compass orientation in migratory birds - PubMed (original) (raw)
A visual pathway links brain structures active during magnetic compass orientation in migratory birds
Dominik Heyers et al. PLoS One. 2007.
Abstract
The magnetic compass of migratory birds has been suggested to be light-dependent. Retinal cryptochrome-expressing neurons and a forebrain region, "Cluster N", show high neuronal activity when night-migratory songbirds perform magnetic compass orientation. By combining neuronal tracing with behavioral experiments leading to sensory-driven gene expression of the neuronal activity marker ZENK during magnetic compass orientation, we demonstrate a functional neuronal connection between the retinal neurons and Cluster N via the visual thalamus. Thus, the two areas of the central nervous system being most active during magnetic compass orientation are part of an ascending visual processing stream, the thalamofugal pathway. Furthermore, Cluster N seems to be a specialized part of the visual wulst. These findings strongly support the hypothesis that migratory birds use their visual system to perceive the reference compass direction of the geomagnetic field and that migratory birds "see" the reference compass direction provided by the geomagnetic field.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
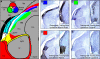
Figure 1. Neuronal tracing reveals that Cluster N receives input through the thalamofugal visual pathway.
A: Schematic side view of the bird's brain indicating the locations of tracer application. Retrograde tracer (BDA, shown in green) was iontophoretically applied into Cluster N (shown in magenta). Anterograde tracer (CtB, shown in red) was injected into the vitreous of the contralateral eye. B: Double-labeling of ZENK and the retrograde tracer BDA in sagittal brain sections at the level of Cluster N proves the correct placement of tracer into Cluster N: arrows point to examples of neurons displaying ZENK-immunoreactivity (shown in magenta) in the nucleus together with BDA (shown in green) in the somata. Scale bar: 25 µm. C: Tracer distribution in frontal brain sections at the level of the thalamic Gld. Anterogradely labeled fibers from the retina (shown in red) project upon all substructures of the Gld, i.e. LdOPT, SpRt and lateral/ventral parts of the DLL. Retrogradely labeled neurons projecting upon Cluster N (visualised green) mainly originate within the DLL, with few additional connections from the LdOPT and SpRt. Scale bar: 50 µm. D: Confocal 3D-stacks in the thalamic Gld at high magnification indicate direct contact (arrows) between retinofugal fibers (shown in red) and somata/proximal dendrites retrogradely labeled from Cluster N (shown in green). Scale bar: 4 µm. Abbreviations: DLL, Nucleus dorsolateralis anterior thalami, pars lateralis; Gld, dorsolateral geniculate complex; LdOPT, Nucleus lateralis dorsalis nuclei optici principalis thalami; Rt, Nucleus rotundus; SPC, Nervus superficialis parvocellularis; SpRt, Nucleus suprarotundus; TSM, Tractus septomesencephalicus.
Figure 2. Cluster N is linked to a specific subsystem of the thalamofugal visual pathway.
A: Schematic overview of anatomical features according to cresylviolet staining (shown in B, C and D) and tracing patterns in the thalamus. Each input source (retina, red; medial wulst, blue; Cluster N, green) and their overlapping tracing patterns are color-coded separately. Retinofugal fibers show a high degree of overlap with neurons projecting upon Cluster N (shown in yellow) in ventral parts of the DLL, the SpRt and the LdOPT, whereas neurons projecting upon the medial wulst exclusively co-localize with retinofugal fibers only in dorsal DLL regions and the DLAmc (shown in magenta). Lateral parts of the DLL, which receive afferents from the retina, project upon the medial wulst and Cluster N (shown in white). B: Retrograde tracing pattern from the medial visual wulst, ipsilateral side. Neurons are mainly located in lateral parts of the DLL and LdOPT with few single neurons positioned in lateral parts of the DLAmc. C: Retrograde tracing pattern from Cluster N, ipsilateral side. Labeled neurons cover lateral and ventral parts of the DLL and are found in the SpRT and LdOPT (compare Fig. 1C). D: Contralaterally projecting retinofugal fibers innervate the LdOPT, SpRt and lateral/ventral parts of the DLL, the nMOT and a small band along the lateral DLL reaching parts of the DLAmc (compare Fig. 1C). E: Retrograde tracing pattern from Cluster N, contralateral side. Few scattered neurons are found in DLL and LdOPT. Scale bar (for B–E): 100 µm; scale bar in insert (for insert in B–E): 50 µm. Abbreviations: AL, Ansa lenticularis; DLAmc, Nucleus dorsolateralis anterior thalami, pars magnocellularis: DLL, Nucleus dorsolateralis anterior thalami, pars lateralis; FPL, Fasciculus prosencephali lateralis; LdOPT, Nucleus lateralis dorsalis nuclei optici principalis thalami; nMOT, Nucleus marginalis tractus optici; OM, Tractus occipitomesencephalicus; Rt, Nucleus rotundus; SPC, Nervus superficialis parvocellularis; SpRt, nucleus suprarotundus; Tel, Telencephalon; TrO, Tractus opticus; TSM, Tractus septomesencephalicus.
Figure 3. Detailed quantification of ZENK protein expression and comparison with ZENK mRNA expression within Cluster N.
A: Expression of ZENK mRNA during night-time covers posterolateral parts of the hyperpallium and underlying mesopallium. In the DNH nucleus, the amount of ZENK mRNA transcripts is decreased. D: Expression of ZENK protein during night-time covers hyperpallial compartments comparable to the expression of ZENK mRNA but decreases in mesopallial portions. Within Cluster N, approximately 56% of neurons show nuclear expression of ZENK protein with highest relative amounts of ZENK-positive nuclei found in the shell surrounding the DNH nucleus. B: Decreased expression of ZENK mRNA and E: protein during day in the whole hyperpallium. Nuclear ZENK protein is found in approximately 22% of Cluster N neurons. Note that, ventral mesopallial (MV) and nidopallial (N) portions show increased ZENK expression on the mRNA and protein level compared to night-time activation patterns. C: Corresponding Nissl-stained section and F: schematic drawing display morphological features and neuroanatomical location of Cluster N within the telencephalon. G: Determination of four subregions within Cluster N defined by morphological boundaries (compare Fig. 3C): DNH nucleus (Fig. 3G, shown in blue); the shell surrounding DNH nucleus (Fig. 3G, shown in green); the remaining hyperpallial Cluster N part (Fig. 3G, shown in yellow); the mesopallial Cluster N part (Fig. 3G, shown in red). Scale bar (for A–G): 250 µm. H: Quantification of percentages of neurons with nuclear expression of ZENK within each subunit. Abbreviations: DNH, dorsal nucleus of the hyperpallium; H, hyperpallium, MD, dorsal mesopallium; MV, ventral hyperpallium; N, nidopallium.
Similar articles
- Calibration of magnetic and celestial compass cues in migratory birds--a review of cue-conflict experiments.
Muheim R, Moore FR, Phillips JB. Muheim R, et al. J Exp Biol. 2006 Jan;209(Pt 1):2-17. doi: 10.1242/jeb.01960. J Exp Biol. 2006. PMID: 16354773 Review. - Migratory birds use head scans to detect the direction of the earth's magnetic field.
Mouritsen H, Feenders G, Liedvogel M, Kropp W. Mouritsen H, et al. Curr Biol. 2004 Nov 9;14(21):1946-9. doi: 10.1016/j.cub.2004.10.025. Curr Biol. 2004. PMID: 15530397 - Visual but not trigeminal mediation of magnetic compass information in a migratory bird.
Zapka M, Heyers D, Hein CM, Engels S, Schneider NL, Hans J, Weiler S, Dreyer D, Kishkinev D, Wild JM, Mouritsen H. Zapka M, et al. Nature. 2009 Oct 29;461(7268):1274-7. doi: 10.1038/nature08528. Nature. 2009. PMID: 19865170 - Night-time neuronal activation of Cluster N in a day- and night-migrating songbird.
Zapka M, Heyers D, Liedvogel M, Jarvis ED, Mouritsen H. Zapka M, et al. Eur J Neurosci. 2010 Aug;32(4):619-24. doi: 10.1111/j.1460-9568.2010.07311.x. Epub 2010 Jul 6. Eur J Neurosci. 2010. PMID: 20618826 Free PMC article. - The magnetic map sense and its use in fine-tuning the migration programme of birds.
Heyers D, Elbers D, Bulte M, Bairlein F, Mouritsen H. Heyers D, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Jul;203(6-7):491-497. doi: 10.1007/s00359-017-1164-x. Epub 2017 Apr 1. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28365788 Review.
Cited by
- Multiple Visual Field Representations in the Visual Wulst of a Laterally Eyed Bird, the Zebra Finch (Taeniopygia guttata).
Bischof HJ, Eckmeier D, Keary N, Löwel S, Mayer U, Michael N. Bischof HJ, et al. PLoS One. 2016 May 3;11(5):e0154927. doi: 10.1371/journal.pone.0154927. eCollection 2016. PLoS One. 2016. PMID: 27139912 Free PMC article. - Cryptochromes--a potential magnetoreceptor: what do we know and what do we want to know?
Liedvogel M, Mouritsen H. Liedvogel M, et al. J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S147-62. doi: 10.1098/rsif.2009.0411.focus. Epub 2009 Nov 11. J R Soc Interface. 2010. PMID: 19906675 Free PMC article. - Magnetoreception through cryptochrome may involve superoxide.
Solov'yov IA, Schulten K. Solov'yov IA, et al. Biophys J. 2009 Jun 17;96(12):4804-13. doi: 10.1016/j.bpj.2009.03.048. Biophys J. 2009. PMID: 19527640 Free PMC article. - Broadband 75-85 MHz radiofrequency fields disrupt magnetic compass orientation in night-migratory songbirds consistent with a flavin-based radical pair magnetoreceptor.
Leberecht B, Kobylkov D, Karwinkel T, Döge S, Burnus L, Wong SY, Apte S, Haase K, Musielak I, Chetverikova R, Dautaj G, Bassetto M, Winklhofer M, Hore PJ, Mouritsen H. Leberecht B, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022 Jan;208(1):97-106. doi: 10.1007/s00359-021-01537-8. Epub 2022 Jan 12. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022. PMID: 35019998 Free PMC article. - Seasonally Changing Cryptochrome 1b Expression in the Retinal Ganglion Cells of a Migrating Passerine Bird.
Nießner C, Gross JC, Denzau S, Peichl L, Fleissner G, Wiltschko W, Wiltschko R. Nießner C, et al. PLoS One. 2016 Mar 8;11(3):e0150377. doi: 10.1371/journal.pone.0150377. eCollection 2016. PLoS One. 2016. PMID: 26953690 Free PMC article.
References
- Wiltschko W, Wiltschko R. The magnetic compass of European Robins. Science. 1972;176:62–64. - PubMed
- Wiltschko R, Wiltschko W. Magnetic Orientation in Animals. Berlin, Heidelberg, New York: Springer Verlag 1995
- Cochran WW, Mouritsen H, Wikelski M. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304:405–408. - PubMed
- Walker MM, Diebel CE, Cordula VH, Pankhurst PM, Montgomery JC, et al. A nose for north? The vertebrate magnetic sense. Nature. 1997;390:371–376. - PubMed
- Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11:462–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources