Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer - PubMed (original) (raw)
Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer
Shojiro Kitajima et al. PLoS One. 2007.
Abstract
Background: The serine/threonine kinase Aurora-A (Aur-A) is a proto-oncoprotein overexpressed in a wide range of human cancers. Overexpression of Aur-A is thought to be caused by gene amplification or mRNA overexpression. However, recent evidence revealed that the discrepancies between amplification of Aur-A and overexpression rates of Aur-A mRNA were observed in breast cancer, gastric cancer, hepatocellular carcinoma, and ovarian cancer. We found that aggressive head and neck cancers exhibited overexpression and stabilization of Aur-A protein without gene amplification or mRNA overexpression. Here we tested the hypothesis that aberration of the protein destruction system induces accumulation and consequently overexpression of Aur-A in cancer.
Principal findings: Aur-A protein was ubiquitinylated by APC(Cdh1) and consequently degraded when cells exited mitosis, and phosphorylation of Aur-A on Ser51 was observed during mitosis. Phosphorylation of Aur-A on Ser51 inhibited its APC(Cdh1)-mediated ubiquitylation and consequent degradation. Interestingly, constitutive phosphorylation on Ser51 was observed in head and neck cancer cells with protein overexpression and stabilization. Indeed, phosphorylation on Ser51 was observed in head and neck cancer tissues with Aur-A protein overexpression. Moreover, an Aur-A Ser51 phospho-mimetic mutant displayed stabilization of protein during cell cycle progression and enhanced ability to cell transformation.
Conclusions/significance: Broadly, this study identifies a new mode of Aur-A overexpression in cancer through phosphorylation-dependent inhibition of its proteolysis in addition to gene amplification and mRNA overexpression. We suggest that the inhibition of Aur-A phosphorylation can represent a novel way to decrease Aur-A levels in cancer therapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
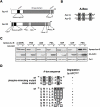
Figure 1. Aur-A overexpression in head and neck cancer may be caused by the abnormality of degradation.
A: Immunohistochemical expression of Aur-A is shown in normal oral mucosa and head and neck cancer. B: Comparison of gene amplification, mRNA expression and protein expression in 6 head and neck cancer cell lines. Gene amplification and mRNA expression were previously examined (9). Protein expression was examined by Western blot analysis. Cul1 expression was used as a loading control. C: Accumulation of Aur-A protein by proteasome inhibitor, ZLLL. Cancer cells were treated with or without 25 µM ZLLL for 6 h. Expression of Aur-A was examined by Western blot analysis. Cul1 expression was used as a loading control. D: Half-life of Aur-A in cancer cells. Cancer cells were treated with CHX for indicated time. Expression of Aur-A was examined by Western blot analysis. Time zeros were normalized for equal amounts of Aur-A rather than equal amount of protein extracts to directly compare the two half-lives. Cul1 expression was used as a loading control.
Figure 2. Phosphorylation on Ser51 inhibits APCCdh1-mediated degradation.
A: FLAG-tagged Aur-A and Xpress-tagged Aur-B were co-transfected with or without HA-tagged Cdc20 or Cdh1 in 293T cell. B: Schematic domain structure of Aur-A wild type (wt) and two deletion mutants (ΔN and ΔC) are shown. The position of two degradation motifs, A-box and D-box, are indicated. C: Aur-A-ΔN or -ΔC mutant was co-transfected with Cdh1 in 293T cell. D: A-box mutated (46RVL48 ->AVA) or D-box mutant (371RPML374 ->APMA) Aur-A was co-transfected with or without Cdh1. E: Ser51 was replaced by alanine (S51A) or aspartic acid (S51D). Each wt, S51A and S51D mutant Aur-A was co-transfected with or without Cdh1 or Cdc20. F: Sensitivity of ubiquitylation of Aur-A wt and S51 mutants were assayed in vitro. APC immunoprecipitated with anti-Cdc27 antibody from the HeLa cell lysates was subjected to the in vitro ubiquitylation assay as described in Materials and methods. The reaction was terminated at 60 min. IVT-Aur-A (arrow) was used as a substrate. “Aur-AUb” indicates ubiquitylated Aur-A.
Figure 3. Aur-B is not degraded by APCCdh1 through mimicry of phosphorylation at Glu32 in A-box.
A: Comparison of schematic structure between Aur-A and -B is shown. Aur-B has several degradation motifs similarly to Aur-A. B: Corresponding amino acid sequence of A-box between Aur-A and -B is shown. C: Aur-B with mutated amino acids in A-box (31KEP33 ->PSN, ASN, PSA, KSP, KAP and PEN) was co-transfected with or without Cdh1. D: Summary of mutated sites and their results are shown.
Figure 4. Phosphorylation on Ser51 during mitosis.
A: Characterization of phosopho-specific antibody against Ser51 of Aur-A. Expression of Ser51 phosphorylated Aur-A protein is examined by immunoprecipitation (IP) with a phosopho-specific antibody against Ser51 of Aur-A followed by immunoblottoing (IB) analysis with a monoclonal antibody to Aur-A in wt and S51 mutants of Aur-A transfected 293T cells. B: Phosphorylation of Ser51 in HeLa cells with or without Noc treatment. C: Phosphorylation on Ser51 in HeLa cells. HeLa cells were released from Noc-induced prometaphase arrest and collected at the indicated times. Samples were analyzed by SDS-PAGE followed by Western blotting with phospho-S51 Aur-A, Aur-A, phospho-T288 Aur-A, phospho-histone H3 (Ser10) and Cul1 antibodies (upper panel). Graph shows expression level of Aur-A, phospho-S51 Aur-A, phospho-T288 Aur-A and phospho-histone H3 (Ser10) (lower panel). D: Expression of Ser51 phosphorylated Aur-A protein is examined by western blot analysis in S51D and K/R (kinase inactive) mutants transfected 293T cells. Phosphorylation on Thr288 was examined to demonstrate that K/R affected as a dominant negative. E: Expression of Ser51 phosphorylated Aur-A protein is examined by western blot analysis in wt and K/R mutant transfected 293T cells with nocodazole (noc) and okadaic acid (OA).
Figure 5. Aur-A overexpression in head and neck cancer cells is caused by phosphorylation on Ser51.
A: Phosphorylation on Ser 51 in head and neck cancer cells. Expression of Ser51 phosphorylated Aur-A protein is examined by immunoprecipitation (IP) with a phosopho-specific antibody against Ser51 of Aur-A followed by immunoblottoing (IB) analysis with a monoclonal antibody to Aur-A in head and neck cancer cells. Gene amplification and mRNA expression were previously examined . B: Constitutive phosphorylation on Ser 51 in head and neck cancer cells. Indicated cancer cell lines were released from noc-induced prometaphase arrest and collected in 4 h. Cells had almost completely exited from mitosis. Expression of Ser51 phosphorylated Aur-A protein is examined by immunoprecipitation (IP) with a phosopho-specific antibody against Ser51 of Aur-A followed by immunoblottoing (IB) analysis with a monoclonal antibody to Aur-A. Cul1 was used as a loading control and phospho-histone H3 (Ser10) was used as a marker for mitosis. C: Expression of Ser51 phosphorylated Aur-A protein is examined by immunoprecipitation (IP) with a phosopho-specific antibody against Ser51 of Aur-A followed by immunoblottoing (IB) analysis with a monoclonal antibody to Aur-A in cells in normal oral mucosal tissue and 9 head and neck cancer tissues. ß-Actin expession was used as a loading control.
Figure 6. S51D mutant Aur-A enhanced cell transformation in comparison with wild type.
A: Alteration of wt and S51D Aur-A expression after nocodazole release in HeLa cells. HeLa cells were transiently transfected with wt and S51D Aur-A. After 48 h of transfection, cells were synchronized by noc arrest and mitotic shake-off, released into fresh medium, harvested at the indicated times. Samples were analyzed by SDS-PAGE followed by Western blotting with FLAG, cyclin-A, p27, phospho-histone H3 (Ser10) and Cul1 antibodies. B: Half-life of wt and mutants (S51A, S51D and KR) of Aur-A transfected 293T cells (left panel). Cells were treated with CHX for indicated time. Right panel shows Aur-A/Cul1 ratio measured by densitometry. C: Effect of S51D mutant Aur-A expression on cell transformation in BALB/c 3T3 A31-1-1 cells. FLAG-tagged wt, S51A and S51D mutants Aur-A were transfected with or without H-Ras (G12V). Expression of wt, S51A and S51D mutants Aurora-A and H-Ras are confirmed by Western blot analysis (left panel). After 2 weeks of culture, the dishes were fixed with ethanol and stained with Giemsa solution (middle panel). Quantification of the number of transformed foci as determined using standard criteria (right panel). Error bars represent the s.d.
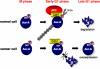
Figure 7. Schematic model of Aur-A overexpression in cancer.
During mitosis, Aur-A is phosphorylated on Ser51 in normal cells. At mitotic exit, Aur-A is de-phosphorylated by PP2A and ubiquitylated by APCCdh1. On the other hand, Aur-A is constitutively phosphorylated on Ser51 in cancer cells. Therefore, Aur-A can not be ubiquitylated and consequently accumulated in cancer cells.
Similar articles
- Oncogenic role of nuclear accumulated Aurora-A.
Tatsuka M, Sato S, Kanda A, Miki T, Kamata N, Kitajima S, Kudo Y, Takata T. Tatsuka M, et al. Mol Carcinog. 2009 Sep;48(9):810-20. doi: 10.1002/mc.20525. Mol Carcinog. 2009. PMID: 19204928 - Requirements for the destruction of human Aurora-A.
Crane R, Kloepfer A, Ruderman JV. Crane R, et al. J Cell Sci. 2004 Dec 1;117(Pt 25):5975-83. doi: 10.1242/jcs.01418. Epub 2004 Nov 9. J Cell Sci. 2004. PMID: 15536123 - The mitotic kinase Aurora-A induces mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway.
Wang LH, Xiang J, Yan M, Zhang Y, Zhao Y, Yue CF, Xu J, Zheng FM, Chen JN, Kang Z, Chen TS, Xing D, Liu Q. Wang LH, et al. Cancer Res. 2010 Nov 15;70(22):9118-28. doi: 10.1158/0008-5472.CAN-10-1246. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045147 - Aurora kinases link chromosome segregation and cell division to cancer susceptibility.
Meraldi P, Honda R, Nigg EA. Meraldi P, et al. Curr Opin Genet Dev. 2004 Feb;14(1):29-36. doi: 10.1016/j.gde.2003.11.006. Curr Opin Genet Dev. 2004. PMID: 15108802 Review. - Aurora A, meiosis and mitosis.
Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Crane R, et al. Biol Cell. 2004 Apr;96(3):215-29. doi: 10.1016/j.biolcel.2003.09.008. Biol Cell. 2004. PMID: 15182704 Review.
Cited by
- Protein tyrosine phosphatase receptor delta acts as a neuroblastoma tumor suppressor by destabilizing the aurora kinase A oncogene.
Meehan M, Parthasarathi L, Moran N, Jefferies CA, Foley N, Lazzari E, Murphy D, Ryan J, Ortiz B, Fabius AW, Chan TA, Stallings RL. Meehan M, et al. Mol Cancer. 2012 Feb 5;11:6. doi: 10.1186/1476-4598-11-6. Mol Cancer. 2012. PMID: 22305495 Free PMC article. - FBXL7 Body Hypomethylation Is Frequent in Tumors from the Digestive and Respiratory Tracts and Is Associated with Risk-Factor Exposure.
Camuzi D, Buexm LA, Lourenço SQC, Grazziotin R, Guaraldi S, Valverde P, Rapozo D, Brooks JM, Mehanna H, Ribeiro Pinto LF, Soares-Lima SC. Camuzi D, et al. Int J Mol Sci. 2022 Jul 15;23(14):7801. doi: 10.3390/ijms23147801. Int J Mol Sci. 2022. PMID: 35887149 Free PMC article. - Aurora kinases in head and neck cancer.
Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Mehra R, et al. Lancet Oncol. 2013 Sep;14(10):e425-35. doi: 10.1016/S1470-2045(13)70128-1. Lancet Oncol. 2013. PMID: 23993387 Free PMC article. Review. - Ubiquitin-Mediated Degradation of Aurora Kinases.
Lindon C, Grant R, Min M. Lindon C, et al. Front Oncol. 2016 Jan 18;5:307. doi: 10.3389/fonc.2015.00307. eCollection 2015. Front Oncol. 2016. PMID: 26835416 Free PMC article. Review. - Non-centrosomal TPX2-Dependent Regulation of the Aurora A Kinase: Functional Implications for Healthy and Pathological Cell Division.
Garrido G, Vernos I. Garrido G, et al. Front Oncol. 2016 Apr 15;6:88. doi: 10.3389/fonc.2016.00088. eCollection 2016. Front Oncol. 2016. PMID: 27148480 Free PMC article. Review.
References
- Murray AW. Recycling the Cell Cycle: Cyclins Revisited. Cell. 2004;116:221–234. - PubMed
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. - PubMed
- Marumoto T, Zhang D, Saya H. Aurora-A-a Guardian of poles. Nat Rev Cancer. 2005;5:42–50. - PubMed
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37-CA76584/CA/NCI NIH HHS/United States
- R01-GM57587/GM/NIGMS NIH HHS/United States
- R01 GM057587/GM/NIGMS NIH HHS/United States
- R21 CA125173/CA/NCI NIH HHS/United States
- R37 CA076584/CA/NCI NIH HHS/United States
- R21-CA125173/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous