Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers - PubMed (original) (raw)
doi: 10.1186/1750-1326-2-18.
Elizabeth Head, Floyd Sarsoza, Tommy Saing, Carl W Cotman, Mihaela Necula, Lawrence Margol, Jessica Wu, Leonid Breydo, Jennifer L Thompson, Suhail Rasool, Tatyana Gurlo, Peter Butler, Charles G Glabe
Affiliations
- PMID: 17897471
- PMCID: PMC2100048
- DOI: 10.1186/1750-1326-2-18
Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers
Rakez Kayed et al. Mol Neurodegener. 2007.
Abstract
Background: Amyloid-related degenerative diseases are associated with the accumulation of misfolded proteins as amyloid fibrils in tissue. In Alzheimer disease (AD), amyloid accumulates in several distinct types of insoluble plaque deposits, intracellular Abeta and as soluble oligomers and the relationships between these deposits and their pathological significance remains unclear. Conformation dependent antibodies have been reported that specifically recognize distinct assembly states of amyloids, including prefibrillar oligomers and fibrils.
Results: We immunized rabbits with a morphologically homogeneous population of Abeta42 fibrils. The resulting immune serum (OC) specifically recognizes fibrils, but not random coil monomer or prefibrillar oligomers, indicating fibrils display a distinct conformation dependent epitope that is absent in prefibrillar oligomers. The fibril epitope is also displayed by fibrils of other types of amyloids, indicating that the epitope is a generic feature of the polypeptide backbone. The fibril specific antibody also recognizes 100,000 x G soluble fibrillar oligomers ranging in size from dimer to greater than 250 kDa on western blots. The fibrillar oligomers recognized by OC are immunologically distinct from prefibrillar oligomers recognized by A11, even though their sizes overlap broadly, indicating that size is not a reliable indicator of oligomer conformation. The immune response to prefibrillar oligomers and fibrils is not sequence specific and antisera of the same specificity are produced in response to immunization with islet amyloid polypeptide prefibrillar oligomer mimics and fibrils. The fibril specific antibodies stain all types of amyloid deposits in human AD brain. Diffuse amyloid deposits stain intensely with anti-fibril antibody although they are thioflavin S negative, suggesting that they are indeed fibrillar in conformation. OC also stains islet amyloid deposits in transgenic mouse models of type II diabetes, demonstrating its generic specificity for amyloid fibrils.
Conclusion: Since the fibril specific antibodies are conformation dependent, sequence-independent, and recognize epitopes that are distinct from those present in prefibrillar oligomers, they may have broad utility for detecting and characterizing the accumulation of amyloid fibrils and fibrillar type oligomers in degenerative diseases.
Figures
Figure 1
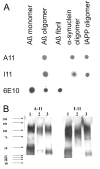
Characterization of OC antiserum. A. ELISA analysis. Plates were coated with homogenous samples of Aβ fibrils, Aβ monomer, Aβ prefibrillar oligomers, and α-synuclein and IAPP fibrils. The samples were reacted with OC serum which indicates that all types of fibrils and not Aβ monomer or prefibrillar oligomers react with OC. B. Dot blot analysis of Aβ42 and polyQ36 prefibrillar oligomers and fibrils. Aβ42 and polyQ fibrils only stain with OC serum, while Aβ42 and polyQ prefibrillar oligomers only react with A11.
Figure 2
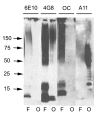
Western blot analysis of Aβ42 fibrils and prefibrillar oligomers. Aβ42 fibrils (F) and prefibrillar oligomers (O) were run on SDS polyacrylamide gels, transferred to nitrocellulose and probed with 6E10, 4G8, OC and A11 antibodies as indicated at the top of the panel. Both fibrillar and prefibrillar oligomer samples contain bands that react with 4G8 ranging from monomer up to the size of material that accumulates at the top of the gel. OC only stains the bands from fibrillar samples of approximately dimer and above. A11 only stains the prefibrillar oligomer samples. 6E10 does not stain prefibrillar Aβ oligomer samples formed at pH 7.4 as previously reported [22].
Figure 3
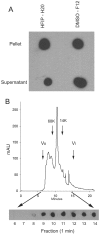
Solubility and size distribution of fibrillar Aβ42 oligomers under physiological conditions. A. Aβ42 aggregates were prepared in HFIP-H20 and DMSO-F12 medium as described in Materials and Methods and centrifuged at 100,000 × G for 1 hr. The supernatant and pellet fractions were separated and the pellet resuspended in an equal volume of PBS. Aliquots of the supernatant and pellet were dotted on nitrocellulose and probed with OC antisera. Both the soluble and insoluble fractions contain significant amounts of OC reactive material. B. Aβ42 aggregates formed in DMSO were fractionated by size exclusion chromatography on a Toso-Haas 2000 SWXL column, 1 minute fractions collected and dotted on nitrocellulose and probed with OC antisera. The elution profile detected by UV absorbance is shown in the top panel. The bottom panel shows the OC immunoreactivity which is detected in fractions from 8–14 minutes, indicating that low MW Aβ oligomers are immunoreactive with OC. The arrows indicate the positions of the void volume (Vo), included volume (Vi) and the elution positions of molecular weight standards.
Figure 4
Comparison of A11 and I11 antibody specificity. A. Dot blots. Aβ monomer, Aβ prefibrillar oligomers (prepared in HFIP-H20, pH 2.5), Aβ fibrils and α-synuclein and IAPP prefibrillar oligomers were spotted on nitrocellulose strips and probed with A11, I11 and 6E10 as a control. A11 and I11 antibodies demonstrate the same specificity for prefibrillar oligomers and do not react with monomer or fibrils. 6E10 stains the Aβ-containing samples, including prefibrillar oligomers formed at acid pH. B. Western blots. Prefibrillar oligomer samples of calcitonin (Lane 1), insulin (Lane 2) and prion peptide 106–126 (Lane 3) were probed with A11 and I11, which give the same staining pattern.
Figure 5
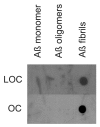
Comparison of LOC and OC antisera specificity. Aβ monomer, prefibrillar oligomers and fibrils were spotted on nitrocellulose strips and probed with LOC and OC antisera. Both LOC and OC react only with fibrillar samples.
Figure 6
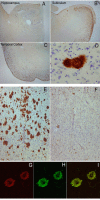
Immunolabeling characteristics of OC in an AD case and preabsorption controls. Extensive OC labeling was observed in the hippocampus (A), subiculum (B) and frontal cortex (C) in AD. A higher magnification photograph illustrates that OC positive deposits were dense and consisted of fine fibrillar material (D). Preabsorption with a 100-fold concentration of OC antigen leads to a significant reduction in immunolabeling in the frontal cortex (E &F). Significant overlap was observed between OC positive immunoreactivity (Red – G) and LOC positive immunoreactivity (Green-H) suggesting that the two antibodies recognize similar deposits (Overlap is yellow – I). Magnification in A, B, C – 1.25×, D – 20×, E, F – 4× and G, H, I – 40×.
Figure 7
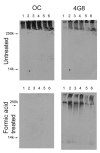
Western blot analysis of Triton X-100 insoluble fractions from human AD brain lysates. Frontal cortex samples, either untreated or formic acid treated, from 6 individual AD brains were dissolved in SDS sample buffer and electrophoresed on polyacrylamide gels and probed with OC or 4G8 as a control. Upper panels: For untreated samples, both OC and 4G8 detect high molecular weight material that accumulates at the top of the gel. Bottom panels: After formic acid treatment, 4G8 detects some lower MW bands in addition to formic acid resistant high MW material, while the staining by OC is abolished, indicating that the staining is conformation dependent.
Figure 8
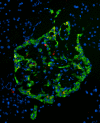
OC staining of pancreatic section from Tg mouse model of type II diabetes expressing human IAPP (obese hemizygous human IAPP transgenic mouse). OC labeling (red) was detected within the islets in insulin-positive (green) beta cells and in the extracellular amyloid deposits.
Figure 9
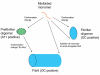
Schematic representation of the distinct types of amyloid oligomers and their relationships to amyloid fibrils. Amyloid aggregation pathways begin with misfolded amyloidogenic monomer (top) and can diverge in two directions depending on which conformation it adopts. It can aggregate to form prefibrillar oligomers by adopting the conformation recognized by A11 (left pathway). These prefibrillar oligomers then align to form protofibrils (not shown) and undergo another conformation change "en bloc" to form fibrils. They are termed prefibrillar oligomers because they are transient intermediates that ultimately become fibrils. Alternatively, amyloidogenic monomer can aggregate to adopt a fibrillar conformation recognized by OC antibody (right pathway). The resulting fibrillar oligomers may represent fibril nuclei which are the minimal stable aggregate that is capable of elongating by recruiting additional monomers. Addition of monomers on to the ends of fibrillar oligomers and fibrils result in fibril growth. The distinction between fibrillar oligomers and fibrils is based on an arbitrary size difference as no conformation difference is apparent. Fibrils may be distinct from fibrillar oligomers on the basis of their content of multiple protofilaments (not shown) but this does not necessarily imply a necessary conformation difference in their integral peptide constituents.
Similar articles
- Fibrillar oligomers nucleate the oligomerization of monomeric amyloid beta but do not seed fibril formation.
Wu JW, Breydo L, Isas JM, Lee J, Kuznetsov YG, Langen R, Glabe C. Wu JW, et al. J Biol Chem. 2010 Feb 26;285(9):6071-9. doi: 10.1074/jbc.M109.069542. Epub 2009 Dec 15. J Biol Chem. 2010. PMID: 20018889 Free PMC article. - Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers.
Kayed R, Canto I, Breydo L, Rasool S, Lukacsovich T, Wu J, Albay R 3rd, Pensalfini A, Yeung S, Head E, Marsh JL, Glabe C. Kayed R, et al. Mol Neurodegener. 2010 Dec 13;5:57. doi: 10.1186/1750-1326-5-57. Mol Neurodegener. 2010. PMID: 21144050 Free PMC article. - Structural classification of toxic amyloid oligomers.
Glabe CG. Glabe CG. J Biol Chem. 2008 Oct 31;283(44):29639-43. doi: 10.1074/jbc.R800016200. Epub 2008 Aug 22. J Biol Chem. 2008. PMID: 18723507 Free PMC article. Review. - Structural features and cytotoxicity of amyloid oligomers: implications in Alzheimer's disease and other diseases with amyloid deposits.
Stefani M. Stefani M. Prog Neurobiol. 2012 Dec;99(3):226-45. doi: 10.1016/j.pneurobio.2012.03.002. Epub 2012 Mar 23. Prog Neurobiol. 2012. PMID: 22450705 Review.
Cited by
- Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques.
Condello C, Yuan P, Schain A, Grutzendler J. Condello C, et al. Nat Commun. 2015 Jan 29;6:6176. doi: 10.1038/ncomms7176. Nat Commun. 2015. PMID: 25630253 Free PMC article. - Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases.
Rinauro DJ, Chiti F, Vendruscolo M, Limbocker R. Rinauro DJ, et al. Mol Neurodegener. 2024 Feb 20;19(1):20. doi: 10.1186/s13024-023-00651-2. Mol Neurodegener. 2024. PMID: 38378578 Free PMC article. Review. - Adaptive downregulation of mitochondrial function in down syndrome.
Helguera P, Seiglie J, Rodriguez J, Hanna M, Helguera G, Busciglio J. Helguera P, et al. Cell Metab. 2013 Jan 8;17(1):132-40. doi: 10.1016/j.cmet.2012.12.005. Cell Metab. 2013. PMID: 23312288 Free PMC article. - Hydrogen sulfide inhibits amyloid formation.
Rosario-Alomar MF, Quiñones-Ruiz T, Kurouski D, Sereda V, Ferreira EB, Jesús-Kim LD, Hernández-Rivera S, Zagorevski DV, López-Garriga J, Lednev IK. Rosario-Alomar MF, et al. J Phys Chem B. 2015 Jan 29;119(4):1265-74. doi: 10.1021/jp508471v. Epub 2015 Jan 15. J Phys Chem B. 2015. PMID: 25545790 Free PMC article. - Accumulation of storage proteins in plant seeds is mediated by amyloid formation.
Antonets KS, Belousov MV, Sulatskaya AI, Belousova ME, Kosolapova AO, Sulatsky MI, Andreeva EA, Zykin PA, Malovichko YV, Shtark OY, Lykholay AN, Volkov KV, Kuznetsova IM, Turoverov KK, Kochetkova EY, Bobylev AG, Usachev KS, Demidov ON, Tikhonovich IA, Nizhnikov AA. Antonets KS, et al. PLoS Biol. 2020 Jul 23;18(7):e3000564. doi: 10.1371/journal.pbio.3000564. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32701952 Free PMC article.
References
- Wisniewski HM, Terry RD. Prog Neuropathol. Vol. 2. 333 ; 1973. Reexamination of the pathogenesis of the senile plaque; pp. 1–26.
- Verkkoniemi A, Somer M, Rinne JO, Myllykangas L, Crook R, Hardy J, Viitanen M, Kalimo H, Haltia M. Variant Alzheimer's disease with spastic paraparesis: clinical characterization. Neurology. 2000;54:1103–1109. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources